1. Introduction
Proteins are complex molecules, and their behavior in mixed aqueous solutions is governed by a number of specific interactions. Amino acids as well as peptides are used such as probe molecules on the way to know the composite nature of protein. The utmost incredible object is that amino acids work not only as originators of hormones, nerves, purphysis pruines, alkaloids, and transition of entire growth then numerous more biomolecules. Amino acids in aqueous solution are ionized and also able to perform as bases or acid. Information on acid-base properties of amino acids is enormously essential in accepting several properties of proteins. The accumulation of solvents/salts in protein solution is well-known to move their configuration and arrangement. Most of the biological and chemical roles of biomolecules take place in aqueous medium. Electrolytes remain estimated to affect water structure, as well as the significance of involvement after structural variations of the solvent to the thermodynamic properties of aqueous solutions of biological molecules takes every so often strained. Amino acids and peptides have been taken up as model compounds for studying the interactions of protein molecules in solutions by a number of researchers [1] - [7] . Amino acids and their derivatives are also known as compensatory (or compatible) solutes in stabilizing proteins and enhancing enzyme activity [8] [9] [10] . The viscosities of L-alanine and L-leucine in 0.5 mol∙L−1 aqueous CdCl2 have been reported by Banipal [2] . Viscosities of glycylglycine have been reported by Badarayani [11] in 0.5 mol∙L−1 aqueous KCl, 0.5 mol∙L−1 aqueous KBr and 0.5 mol∙L−1 aqueous Na2SO4 at 298.15 K and by Santosh [7] in 0.05 mol∙L−1 aqueous MnCl2 at different temperatures. Sadeghi [6] reported viscosities of L-alanine in 0.5 mol∙L−1 aqueous (KH2Cit) and 0.5 mol∙L−1 aqueous (K3Cit). Wadi [12] reported viscosities of alanine in 5 mol∙L−1 aqueous KSCN at different temperatures. Yan [13] reported viscosities of L-alanine and L-leucine in aqueous solution. Viscosities of L-phenylalanine, L-proline, L-glutamic acid and L-leucine in 2.0 mol∙L−1 aqueous NaCl and 2.0 mol∙L−1 aqueous NaNO3 [5] of L-histidine, L-glutamic acid, L-tryptophan, and glycylglycine in 2.0 mol∙L−1 aqueous KCl and 2.0 mol∙L−1 aqueous KNO3 solutions1 and of L-leucine, L-alanine, L-valine and L-proline in 2 mol∙L−1 aqueous KNO3/KCl solutions [14] have been measured for several molal concentrations of amino acids/peptides at different temperatures of (303.15, 308.15, 313.15, 318.15, and 323.15) K earlier in our laboratory. The viscosity B-coefficient measures the size, shape and structural effects, induced by the solute-solvent interactions in solutions [15] . The B-coefficient of the Jones Dole experiential equation of the relative viscosities of electrolyte solutions as a function of their concentration is imperative for numerous reasons: 1) They propose an exceptional basis of main statistics, in that dimension of the viscosity stipulate the uppermost accuracy on the part of the experimentalist. It is in the nature of the dimensions that they effortlessly disclose deprived experimental practices. 2) The B-coefficient is recognized to deliver data relating to the solvation of the ions also their impact on the construction of the solvent in the nearby environment of the solute subdivisions. 3) Owing to the behavior of B-coefficient and their ion preservative properties, possibly significant associations occur amid these coefficients also other ion preservative properties such as Entropies of hydration, Enthalpies, Gibb’s free energies, solvation as well as transfer amongst solvents. In this comprehension, the B-coefficients might afford the vital for the validation of a host thermochemical data. A number of researchers have determined the viscosity B-coefficients of amino acid and peptide molecules in aqueous medium [16] - [21] aqueous electrolytes [22] [23] [24] [25] , and organic solvent systems [26] [27] [28] [29] [30] . As electrolytes are integral part of biological system, hence, the study of such systems may be very useful in understanding the mechanism of drug action that ultimately facilitates the illustration of drug—electrolyte chemistry [31] . This study is focused on the viscometric properties of L-leucine, L-glutamine, L-alanine, and glycylglycine in 0.512 mol·kg−1 aqueous K2SO4/0.512mol∙kg−1 aqueous KNO3 solutions as a function of amino acids/peptides molal concentration at temperatures (298.15, 303.15, 308.15, 313.15, 318.15 and 323.15) K. The measured viscosity data have been used to compute the relative viscosity, specific viscosity, viscosity B-coefficient, activation free energy, activation enthalpy and activation entropy values with the view to understanding the ion-ion, ion-hydrophilic and ion-hydrophobic interactions in the systems.
2. Experimental Section
The amino acids/peptides: L-leucine, L-glutamine, L-alanine, and glycylglycine and the salts: potassium sulphate and potassium nitrate of high purity (by mass fraction ≥ 99%), used in the present studies, were purchased from SRL (India) and E. Merck (India), respectively. The amino acids/peptides were recrystallized twice in (ethanol + water) mixtures, dried at T = 383.15 K and kept in a vacuum desiccator over P2O5 for at least 72 h before use. The salts were recrystallized in triply distilled water, dried at T = 423.15 K for at least 3 h and then kept over P2O5 in a vacuum desiccator at room temperature for a minimum of 48 h prior to their use. Stock solutions of 0.512 mol∙kg−1 aqueous K2SO4 and 0.512 mol∙kg−1 aqueous KNO3 solutions were prepared at 298.15 K in triply distilled water and were used as solvents for the preparation of L-leucine, L-alanine, L-glutamine and glycylglycine solutions. All the solutions were stored in special air tight bottles to prevent the exposure of solutions to air and evaporation.
The viscosity measurements were carried out using a suspended Ubbelohde viscometer (Figure 1), which was calibrated with triply distilled water at six temperatures between (298.15 and 323.15) K. A thoroughly cleaned and perfectly dried viscometer filled with the test solution was placed vertically in the glass walled thermostat maintained at a desired temperature (±0.01 K). After the attainment of thermal equilibrium, efflux times of flow were recorded with an electronic watch with the resolution of 0.01 s. The average of at least four readings reproducible within 0.1 s was used as the final efflux time. The viscosity values of water at different temperatures were taken from the literature for calibration purposes [32] . The viscosity coefficient is defined as the following Poiseuille’s equation,
(1)
where g, h, ρ, r, l and t are acceleration due to gravity, height of the column in the viscometer, density of the liquid, radius of the viscometer’s capillary, length and time of fall for the liquid of volume v through the capillary, respectively.
The above equation can also be written as
(2)
where β = πghr4/8vl is constant for the given viscometer. The viscosity value of the test solution was calculated using the reported viscosity values of pure water at various temperatures.
Equation (3) given below was employed for the calculation of viscosities of solutions.
(3)
where η1, ρ1 and t1 are the viscosity coefficient, density and time of fall of the solution, respectively; whereas the η2, ρ2 and t2 are the viscosity coefficient, density and time of fall of the solvent at the given temperature, respectively. The uncertainty in the measurement of viscosity was ±3 × 10−6 Pa∙s.
3. Results and Discussion
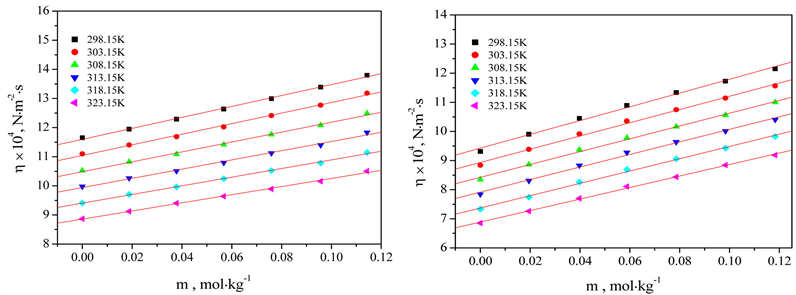
The measured viscosity values of (L-leucine/L-glutamine/L-alanine/glycylglycine + 0.512 mol∙kg−1 aqueous K2SO4/0.512mol∙kg−1 aqueous KNO3) solutions as functions of amino acids/peptides molal concentration and temperature have been presented in Table 1. The viscosity values have been least-squares fitted to the following polynomial,
(4)
where η0, η1, η2; and m are the fitted coefficients and molal concentration of solutions, respectively. The fitted coefficients of Equation (1) have been given in Table 2. The η values of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4/KNO3 solution increase with increase in molal concentration in all the systems under examination.
The relative viscosity [33] [34] [35] and specific viscosity [36] values have been computed by retaining the following relations expending the viscosity values of solvent and solution,
![]()
Table 1. Viscosities (η/10−4, Pa∙s) as functions of amino acids/peptides molal concentration and temperature.
![]()
Table 2. ηo, η1 and η2 as a function of molal concentration of amino acids/peptides at different temperatures.
(5)
(6)
The calculated values of relative and specific viscosity have been registered in Table 3 and Table 4, respectively. The ηr values increase with an increase in molal concentration of amino acids/peptides in 0.512 mol∙kg−1 aqueous K2SO4 and 0.512 mol∙kg−1 aqueous KNO3 solutions but these values do not reveal regular developments of variation with an increase in temperature in the systems under investigation. The reported relative viscosity values of 0.03061 m L-leucine in 0.5 M aqueous magnesium acetate solution at 298.15 K is 1.0055 [37]
![]()
Table 3. Relative viscosity values (ηr) as functions of amino acids/peptides molal concentration and temperature.
![]()
Table 4. Specific viscosity values (ηsp × 102) as functions of amino acids/peptides molal concentration and temperature.
whereas the observed value of 0.0378 mL-leucine in 0.5 M aqueous K2SO4 solution is 1.091. The relative viscosity of 1.0052 m L-alanine in 0.05 M aqueous MgCl2 solution as 1.027 Banipal et al. has been reported by Banipal [38] whereas the detected value of 1.0017 m L-alanine in 0.5 M aqueous K2SO4 solution is 1.092. The increasing trend of ηr values with an increase in solute concentration may be attributed to an increase in the zwitterion-ion (-COO−-K+, -
-
/
) interactions in solutions.
The viscosity data have been fitted to the following Jones-Dole [39] equation,
(7)
where ηr (=η/ηo) is the relative viscosity of the solution; m is the molal concentration of solution; A, the Falkenhagen coefficient, represents the solute-solute interactions; and B-coefficient reflects solute-solvent interactions [40] [41] . The B-coefficient values along with the standard deviations of linear regression have been listed in Table 5. The observed and the literature values of B-coefficients of the studied amino acids and peptides have been listed in Table 6 for comparison purpose. The B-coefficients of the amino acids and peptides reflect the net structural effects of the charged end groups (
, COO–) and the hydrophobic CH2 groups on the solvent. The contribution of CH2 groups in B-coefficient values varies with the number of carbon atoms in their alkyl chain at a given temperature. These two effects can be separated by noting the linear relationship [42] [43] of B-coefficient with the number of carbon atoms, nc,
(8)
![]()
Table 5. Viscosity A-coefficients (molal−1/2) values at different temperatures.
![]()
Table 6. Viscosity B-coefficients (dm3∙mol−1) and (dB/dT) values at different temperatures.
The B-coefficients of L-leucine, L-glutamine, L-alanine and glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 and 0.512 mol∙kg−1 aqueous KNO3 solutions are positive at all temperatures of study. The temperature derivative of B-coefficient values (dB/dT) has been presented in Table 6. The positive values of B-coefficients of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions indicate the possibility of a strong alignment of zwitterions with ions/water dipoles [44] [45] [46] . The observed higher B-coefficients values of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions than those in the aqueous medium may be attributed to K+-
, K+-
and -COO−-K+, -
-
/
interactions operative in the solutions. The positive B-coefficients along with negative (dB/dT) values of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions indicate the structure making nature of solute in the solutions [41] [47] [48] . The trend of variation of viscosity B-coefficients of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions is found to be as:
L-leucine > L-glutamine > glycylglycine > L-alanine
The largest B-coefficient of L-leucine can be attributed to the domination of ion (K+/
/
) -ion (-COO−/
) interactions and ion-hydrophobic interactions between ions K+/
/
and hydrophobic group (-CH3-CH2-CH-CH3) of L-leucine over the ion-ion and ion-hydrophilic interactions between ions K+/
/
and hydrophilic group (-CONH2) of L-glutamine. The ion (K+/
/
) -ion(-COO−/
) interactions and ion-hydrophilic interactions between ions K+/
/
and hydrophilic group (-CONH2) of L-glutamine over the ion-ion and ion-hydrophilic interactions between ions K+/
/
and hydrophilic group -CONH of glycylglycine. The ion-hydrophilic interactions are absent in L-alanine—aqueous K2SO4/KNO3 system. The ion-ion interactions and ion-hydrophobic group interactions of (L-leucine-aqueous salts) dominate over ion-hydrophilic interactions in (L-glutamine-aqueous salts)/(glycylglycine-aqueous salt) systems. In the case of L-alanine, the ion (K+/
/
) -ion (-COO−/
) interactions dominate over ion (K+/
/
) -hydrophobic group (-CH3/-CH2) interactions. The B-coefficients of studied amino acids and peptides in 0.512 mol·kg−1 aqueous K2SO4 and 0.512 mol∙kg−1 aqueous KNO3 solutions decrease with an increase in temperature thereby showing that the zwitterion-ion interactions further weakened with the increase in temperature. The observed and literature values of viscosity have been mentioned in Table 7.
![]()
Table 7. Observed and literature values of viscosity at 298.15 K.
Thermodynamic activation parameters of viscous flow of the amino acids/peptides in 0.512 mol∙kg−1 aqueous K2SO4/KNO3 solution have been evaluated by Feakins [49] extension of Eyring transition-state theory,
(9)
where
and
are the standard partial molar volumes of the solvent and solute, respectively.
is the contribution per mole of solvent to the free energy of activation for viscous flow of the solution, and is given by
(10)
where h is the Planck constant, NA is Avogadro’s number, ηo is the viscosity of the solvent, R is the gas constant and
is the contribution per mole of solute to the free energy of activation for viscous flow of the solution and Equation (9) can be rearranged as follows:
![]()
Table 8.
,
,
,
, entropy,
for amino acids/peptides solutions at different temperatures.
(11)
where
is the mean volume of the solvent. The terms xi and Mi denote the mole fractions and molecular weights of water and K2SO4/KNO3. The calculated values of
,
and
are given in Table 8. The positive and large value of
than
indicates that amino acids/peptides-solvent interaction in the ground state is stronger than in the transition state. This means that the formation of the transition state is accompanied by the rupture and distortion of the intermolecular forces in the solvent structure. Thus, amino acids/peptides molecules are acting as structure-makers. This is consistent with the conclusion obtained from B-coefficients. The structure making nature of L-leucine and L-alanine in 0.05 mol·L−1 aqueous magnesium chloride has been reported by Lark [50] and L-glutamine in 0.05 mol·L−1 aqueous tetramethylammonium bromide by Anwar [51] . According to transition state theory [12] every solvent molecule in one mole of solution must pass through the transition state and interact more or less strongly with solute molecules. The positive
values of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions increase with an increase in temperature which suggests that the formation of transition state is less favoured in the presence of L-leucine, L-glutamine, L-alanine and glycylglycine. This is due to the breaking and distortion of intermolecular bonds, which effectively means that more solute-solvent bonds must be broken to form the transition state. Similar results for α-amino acids in aqueous sodium acetate solutions have been reported by Feakins [52] .
The entropy and enthalpy of activation for viscous flow of the amino acids/peptides have been calculated by
(12)
(13)
The
and
values at different temperatures are given in Table 8. The positive
and
values for all studied amino acids/peptides at each temperature of study indicate the formation of the activated complex which is associated with bond breaking and a decrease in order. This suggests that the ground states are in the ordered region.
4. Conclusion
The B-coefficient of L-leucine, L-glutamine, L-alanine and glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 and 0.512 mol∙kg−1 aqueous KNO3 solutions are found to be positive at all temperatures of study. The trend of variation of viscosity B-coefficients of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4 or 0.512 mol∙kg−1 aqueous KNO3 solutions is found to be as: L-leucine > L-glutamine > glycylglycine > L-alanine. The variations of the trend have been discussed in terms of ion-ion, hydrophilic-ion and hydrophobic-ion interactions operative in the systems. The free energy of activation per mole of solute,
reinforce the structure-making ability of L-leucine/L-glutamine/L-alanine/glycylglycine in 0.512 mol∙kg−1 aqueous K2SO4/KNO3 solutions. The effect of solute size on the B-coefficient is outward as of solvodynamic models. A “structure-modelling” solute sinks the usual operative kinetic energy of the solvent particles and thus rises the viscosity of the solution, then gives rise to high B-coefficient values. Due to the exponential affiliation amid viscosity and temperature, an increase in temperature of the solution as a whole causes B-coefficient to decrease. Such efforts have been used to classify “structure creation” solutes. On the contrary, “structure flouting” solutes have relatively low B-coefficients, which upsurge with a rise in temperature. In the case of electrolytes, the B-coefficient is a quantity of the demand or disorder acquainted with ions into their co-ranges. Viscometric studies of amino acids in salts are also important for variety of applications including food science where it is helpful to study the behaviour of food ingredients and effect of salts on the texture and quality of food products, as well as in environmental science to study the behaviour of amino acids in natural water and their interactions with salts which can provide information on the environmental fate of amino acids. In the field of Industrial chemistry, it can be used to analyse the behaviour of surfactants and other chemicals in industrial process and to optimize their performance.
Acknowledgements
The authors are thankful to the Chairman Department of Chemistry, A. M. U. Aligarh for providing the necessary facility for the compilation of this work. Financial support from the UGC-SAP (DRS-I) is acknowledged.
Appendix