Oxone-Mediated Preparation of Ester Derivatives Using Indium(III) Triflate and Various Alcohols ()
1. Introduction
Esters are popular as one of the most fundamental chemical moieties, being composed of a combination of carboxylic acids and alcohols. Among esters, methyl esters are often primarily utilized for protecting the functionality of carboxylic acids because leaving carboxylic acids in their unprotected free forms could cause unwanted side reactions upon implementing certain chemical reactions. Meanwhile, in the fields of medication and chemotherapy, a dose of medicines that possesses ester moieties can often serve as prodrugs with improved bioavailability. Due to enhanced lipophilicity by esterification on a parent drug, improved pharmacological properties such as increased oral and transdermal absorption would be expected. Esters are generally subjected to hydrolysis in their active form in the body by way of the function of esterase. As an example of esters, osertamivir, one of the anti-influenza agents, has the structure of ethyl ester to raise oral bioavailability, which is followed by cleavage into the active form of a carboxylate [1]. Also, dexamethasone 17-valerate and dexamethasone 17,21-dipropionate are formulated agents in dermatological medication to treat skin inflammation, for example. Their topical efficacy is promoted through the form of valerate and propionate esters respectively that enhance transdermal absorption [2] [3].
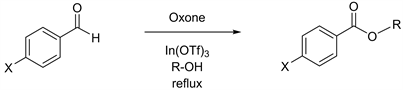
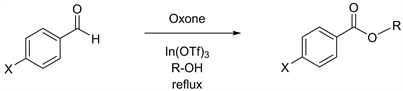
We have been conducting research on indium and indium related reagents and have reported an efficient method of high-yielding methyl esterification, which utilizes trivalent InCl3 under mild reaction conditions [4]. Other examples of the preparation of methyl esters include a reaction by stirring carboxylic acids in methanol in the presence of a catalytic amount of trimethylsilyl chloride [5]. Throughout our continuous study, we have developed Oxone-mediated oxidative methyl esterification of benzaldehyde derivatives [6]. The reactions were accelerated in the presence of In(OTf)3, via the use of an effective oxidant, Oxone® monopersulfate compound (Oxone), which is composed of a triple salt of potassium peroxymonosulfate. Oxone is a well-known stable oxidant with versatile features that enable a wide variety of applications [7]. Oxone, as an example, can be an efficient oxidant for the epoxidation of alkenes [8] [9] [10] [11] [12]. In another example, Oxone can function for the conversion from sulfides to sulfones [13] [14]. The use of Oxone with wet-Alumina processes the ring expansion from cyclic ketones to lactones [15], and this kind of ring expansion can also be carried out by using Oxone in the presence of ionic liquids as solvents through the way of Beayer-Villiger oxidation [16]. We then applied Oxone-mediated reactions to heterocyclic aldehydes with the usage of methanol as well as other alcohols having various chain lengths [17]. Ester derivatives are often prepared to increase therapeutic effects of drugs through improved bioavailability. Enhanced lipophilicity of ester derivatives can be indicated to resolve some difficulties in therapeutic procedures. This research now will become a desirable solution to upgrade public health. In the view of these previously reported esterification methodologies [6] [17] [18], we thereafter further explored Oxone-mediated preparation of esters on benzaldehyde and its derivatives as starting materials in the presence of a catalytic amount of In(OTf)3 and investigated alcohols with various chain lengths, which function as solvents and substrates. The details of our studies are presented herein.
2. Results and Discussion
First, we initiated examination of Oxone-mediated preparation of ester derivatives using benzaldehyde as starting material as in Table 1. Applying the reaction condition from our previous work [6] [17], oxidative esterification was carried out in alcohols with elongated carbon chains. The desired products of methyl

![]()
Table 1. Reactions using benzaldehyde and various alcohols.
aAll reactions were carried out in the presence of 1.0 equiv. of Oxone and 10 mol% of In(OTf)3. bIsolated yields.
ester [19] [20], ethyl ester [21], propyl ester [22], and butyl ester [23] were furnished respectively without any experimental difficulties (Table 1, entries 1 - 4). Upon comparing the yields, a gradual increase is observed from 63% in methanol in entry 1 to 86% in butanol in entry 4, which can be occurred in accordance with boiling point raising from methanol to butanol at reflux. However, in the case of heavier alcohols such as pentanol and hexanol, the reactions did not proceed properly, instead, they turned the reaction mixtures into blackened solutions. Perhaps, physical properties, like viscosity and less polarity of these heavier alcohols, can be the cause of these unproductive results.
Having desired yields of benzaldehyde, we next conducted Oxone-mediated reactions starting with benzaldehyde derivatives possessing either electron donating groups or electron withdrawing groups at the C-4 position on the benzene ring. In the case of 4-tolualdehyde in methanol, a good yield of 82% has been reported previously (Table 2, entry 1) [6]. However, further attempts in ethanol, propanol, and butanol gave unsatisfactory yields (Table 2, entries 2 - 4) [23] [24] [25], prompting us to carry out the identical reactions starting with 4-anisaldehyde derivatives that has a methoxy group at the C-4 position. The outcome was similar, the reaction in methanol furnished the product in a good yield of 82% (Table 2, entry 5), but the reactions in ethanol, propanol, and butanol yielded them in less than 10% (Table 2, entries 6 - 8) [23] [24]. Consequently, we further expanded our exploration to benzaldehyde derivatives with electron withdrawing groups at the C-4 position. In the case of 4-chlorobenzal-dehyde, although the reaction in methanol gave a moderate yield of 73% in 3 h
![]()
Table 2. Reactions using benzaldehyde with electron donating groups and various alcohols.
aAll reactions were carried out in the presence of 1.0 equiv. of Oxone and 10 mol% of In(OTf)3. bIsolated yields.
(Table 3, entry 1), the reactions in ethanol, propanol, and butanol afforded the corresponding products in more than 80% yields in slightly longer reaction time (Table 3, entries 2 - 4) [23] [24] [26]. The reactions starting with 4-cyanobenzal-dehyde also proceeded smoothly (Table 3, entries 5 - 8) [27], including excellent yields of 94% in methanol (Table 3, entry 5). Furthermore, the oxidative esterification with 4-nitorobenzaldehyde, which has a very strong electron withdrawing group at the C-4 position, were examined, and it unexpectedly turned out that the reactions in longer carbon chains of propanol and butanol displayed higher productivity of 88% and 96% yields (Table 3, entries 11 and 12) [28] [29] than the yields of 70% and 35% through the reactions in methanol and ethanol (Table 3, entries 9 and 10) [27].
![]()
Table 3. Reactions using benzaldehyde with electron withdrawing groups and various alcohols.
aAll reactions were carried out in the presence of 1.0 equiv. of Oxone and 10 mol% of In(OTf)3. bIsolated yields.
3. Conclusion
In conclusion, we have investigated Oxone-mediated preparation of ester derivatives in the presence of a catalytic amount of In(OTf)3. As starting materials, benzaldehyde and its derivatives were subjected to the reaction conditions with various alcohols. Comparing benzaldehyde derivatives with electron donating groups and ones with electron withdrawing groups at the C-4 position, the reactions overall went on smoothly and produced corresponding esters in higher yields, in the case of benzaldehyde derivatives with electron withdrawing groups than the derivatives with electron donating groups, noticeably upon implementation in alcohols with longer carbon chains.
4. Experimental
4.1. Materials and Instruments
All reagents were of analytical grade purchased commercially and used without further purification. All reactions were performed under argon using magnetic stirring unless otherwise stated. 1H NMR and 13C NMR spectral data were recorded on a JEOL JMTC-500 spectrometer using TMS as the internal standard.
4.2. General Experimental Procedure
Benzaldehyde (5 mmol) as starting material was combined with Oxone (5 mmol) and In(OTf)3 (10 mol%) in methanol (50 mL). The reaction mixture was refluxed and monitored for completion by TLC. The reaction mixture was filtered, and the filtrate was condensed by rotary evaporation. The resulting residue was purified by silica gel flash column chromatography to obtain the desired esters, which were confirmed by spectroscopy [6] [19] - [29].
4-Methoxybenzoic acid propyl ester: 1H NMR (500 MHz, Chloroform-d) δ 8.00 (d, 2H, J = 9.2 Hz), 6.91 (d, 2H, J = 9.2 Hz), 4.24 (t, 2H, J = 6.9 Hz), 3.85 (s, 3H), 1.77 (sext, 2H, J = 7.5 Hz), 1.02 (t, 3H, J = 7.5 Hz); 13C NMR (125 MHz, Chloroform-d) δ 166.4, 163.2, 131.5, 122.9, 113.5, 66.2, 55.3, 22.1, 10.5.
4-Cyanobenzoic acid propyl ester: 1H NMR (500 MHz, Chloroform-d) δ 7.99 (d, 2H, J = 8.6 Hz), 7.61 (d, 2H, J = 8.6 Hz), 4.17 (t, 2H, J = 6.3 Hz), 1.66 (sext, 2H, J = 7.5 Hz), 0.89 (t, 3H, J = 7.45 Hz); 13C NMR (125 MHz, Chloroform-d) δ 164.3, 133.8, 131.7, 129.5, 117.4, 115.7, 66.7, 21.5, 9.9.
4-Cyanobenzoic acid butyl ester: 1H NMR (500 MHz, Chloroform-d) δ 8.11 (d, 2H, J = 8.6 Hz), 7.71 (d, 2H, J = 8.6 Hz), 4.33 (t, 2H, J = 6.6 Hz), 1.73 (quint, 2H, J = 7.5 Hz), 1.45 (sext, 2H, J = 7.5 Hz), 0.95 (t, 3H, J = 7.5 Hz); 13C NMR (125 MHz, Chloroform-d) δ 164.8, 134.2, 132.1, 129.9, 117.9, 116.1, 65.5, 30.5, 19.1, 13.6.