Predictors of Recurrence of Hepatocellular Carcinoma after Transarterial Chemoembolization: Role of Hepatocyte Growth Factor ()
1. Introduction
Hepatocellular carcinoma is the third leading cause of tumor related mortality after tumors of the lung and the stomach [1] and the prevalence is high in subSaharan Africa and Southeast Asia. It develops mostly in patients with chronic liver disease and liver cirrhosis [2]. Multiple treatment option are available but the choice depends on the stage and the patient liver function [3].
Surgical treatment either resection or transplantation remains the mainstay of potentially curative therapy but the prognosis remains poor due to advanced stage of the tumor at time of diagnosis and the recurrence rate at 5 years after resection is about 80% [4].
Transarterial chemoembolization (TACE) is the best therapeutic option in about 60% to 70% of patients with advanced stage of the disease [5], when curative options such as radiofrequency and surgery are unsuccessful or unfeasible TACE could be another option [6]. The long term outcomes of patients treated with TACE are not entirely satisfactory due to high recurrence rates [7].
Several risk factors of recurrence of HCC after TACE have been reported such as number of focal lesions, size of the lesion, tumor markers and liver function [8].
Human hepatocyte growth factor (HGF) is produced in various organs of the body and is characterized as a multifunctional factor with various biologic activities [9], several reports have examined the relationship between HGF and liver diseases and several have sought a factor that would assist in predicting the development of chronic hepatitis (CH), and the facilitation of the occurrence of HCC [10].
HGF exerts its actions through tyrosine phosphorylation of its receptor, cMET, which is a proto-oncogene product. HGF (also known as the scattering factor) activates the MET signaling pathway, thereby increasing the invasive and metastatic potential of the cells and allowing the survival of cancer cells in the blood stream and facilitating cancer progression [11].
In HCC, the mRNA levels of HGF and the MET receptor are markedly increased compared with those in normal liver. A high serum HGF concentration is associated with a poor prognosis for overall survival after hepatic resection, and the serum level of HGF represents the degree of the carcinogenic state in the livers of patients with C-viral chronic hepatitis and cirrhosis [11].
However, the predictive factors for recurrence after complete response (CR) after TACE have not yet been elucidated. Here, we investigated the predictive factors of recurrence specially the role of HGF in patients with HCC treated with TACE.
2. Patients and Methods
Study Design
The present study was a retrospective cohort study performed at a single centre. The medical records of 250 patients treated with TACE as a first treatment according to BCLC Staging (stage B) between June 2017 and June 2018 at Sohag Digestive Diseases center (Sohag, Egypt) were reviewed.
The inclusion criteria for TACE were BCLC B HCC patients with Child’s A or B cirrhosis, patent portal vein and patients willing for therapy and follow up. Some patients of BCLC A who were unsuitable for ablative therapy or surgery were also included.
The exclusion criteria for TACE were: 1) previous intervention; 2) main portal vein thrombosis; 3) hepatic encephalopathy; 4) uncontrolled Ascites; 5) recent attack of gastrointestinal bleeding within past month. Of these, 125 patients achieved complete response 1 month post TACE as documented by triphasic CT liver and subsequently observed over a 1-year period.
Twenty five patients were excluded as they were lost to follow up. So, only 100 patients (72 males, 28 females) were included in final statistics. These patients were divided into two groups according to disease free survival (DFS) status at 1 year: the non early recurrence (NER) group (1) (no recurrence within 1 year after initial TACE; n = 55) and the early recurrence (ER) group (2) (recurrence within 1 year after initial TACE; n = 45) and The study was approved by the local ethics commitee of Sohag Digestive Diseases Centre (Sohag, Egypt) in May 2017 according to the principles of the Declaration of Helsinki 1964 and written informed consent was obtained from all patients before treatment.
Diagnosis of HCC
The diagnosis of HCC was confirmed in patients with chronic liver disease and cirrhosis with typical vascular pattern on dynamic imaging modalities (i.e. Triphasic CT) which showed contrast hyperenhancement in the arterial phase (wash in) and hypoenhancement in the portal venous and/or delayed phase (wash out) without need to liver biopsy. Complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target lesions or compact lipiodol uptake 1 month after procedure according to modified Response Evaluation Criteria in Solid Tumors [7]. The recurrence is determined when a new lesion appeared or when an enhanced part was seen at the margin of the original lesion.
The Procedure of TACE
Definition: Is a dual therapeutic approach involving concomitant hepatic artery embolization and infusion of concentrated dose of chemotherapeutic drugs.
This procedure has several goals:
Prolongation of contact time between the cytotoxic agent and the tumor allows greater concentration of the chemotherapeutic drug in the tumor. (Experimental studies have shown that embolization can triple the local concentration of Adriamycin.)
Embolization induced ischemia that can enhance the effect of chemotherapy and finally, embolization reduces general side effects of cytotoxic drug [12].
Since TACE was introduced as a palliative treatment in patients with unresectable HCC, it has become one of the most common forms of interventional therapy [13].
TACE Technique
Preprocedural preparation:
The patient would be fasting for 6 hours and the groin shaved;
Undergo vigorous intravenous hydration (500 mL 5% dextrose in normal saline solution before chemoembolization then continued at 100 mL/h) for at least 24 hours;
Preprocedural medications include a prophylactic antibiotic and anon-steroidal anti-inflammatory drug (NSAID).
Angiographic procedure:
Selective hepatic angiography through transfemoral Seldinger technique was conducted with a digital angiographic unit (Siemens Artis Zee).
A 5F valved sheath (introducer 11 cm long sheath) was inserted into the femoral artery with the patient under local anesthesia, (10 ml of 2% lidocaine Hcl).
Celiac and superior mesenteric arteriography was performed with Copra II catheter. (Simon II catheter sometimes used).
The catheter was advanced into the common hepatic artery distal to the origin of gastroduodenal artery, by using a 0.035 inch gliding guide wire (Gide wire Terumo). The catheter must be advanced as selective as possible very close to the tumor (micro catheter used in many cases).
Selective hepatic angiography data were recorded with a digital subtraction technique. Then we Inject 1 ml lidocaien mixed with 10 cc saline.
The patients were injected by a mixture of Lipiodol and cytotoxic drugs. (The mixture of injected material consists of Lipiodol and Doxorubicin) was prepared as follows: 50 mg powder of the Adriablastin (adryiamycin hydrochloride) dissolved in a 10 ml saline. The dissolved Adriablastin is mixed with 7 ml iodized oil Lipidol. The remaining 3 ml iodized oil Lipiodol injected later.
The homogeneous manually shacked emulsion of Lipidol and chemotherapeutic agent were injected through the catheter into the tumor vascular bed under screen, until the Lipidol is densely accumulated into the tumor, sometimes this is followed by occlusion of the tumor vascular bed by PVA particles (When we depicted that there is dominant supply to the tumor with almost no side branches).
After complete response was achieved patients were evaluated every 3 months by Triphasic CT for 1 year for assessment of recurrence.
Assessment of Serum HGF Level
Ten milliliters of venous blood was taken from each participant under aseptic conditions and by using sterile disposable gloves and then we divide the samples as follow:
1) 1.8 ml collected in 0.2 sodium citrate for prothrombin time (PT), prothrombin concentration (PC) and international normalized ratio (INR) which was assay by Thrombol-S kit using Sysmex CA-1500 fully automated coagulometer (Siemens Healthcare GmbH, Laboratory Diagnostic, Marburg, Germany).
2) We collected the rest of sample in a plain tube which was left to clot then the serum was separated and divided into three aliquots:
a) The first one was used for assessment of serum creatinine, serum bilirubin, and albumin which were assayed on the same day using Cobas C311 Chemistry Analyser System (Roche Diagnostic GmbH, Indianapolis, IN, USA).
The second and the third aliquots were stored at −20˚C until the time of assay.
b) The second aliquot was used for assessment of alphafetoprotein and serological testing for anti-HCV and HBsAg which were done using architect i1000 system (Abbott Laboratories, Diagnostic Division Abbott Park, IL).
c) The third aliquot was used for assessment of hepatocyte growth factor by quantitative sandwich ELISA technique using HGF ELISA kit catalog No. DHG00. R&D Systems Inc., Minneapolis, Minnesota, USA (using ELISA Thermo Fisher, Scientific Multiscan EX Microplate Reader, OY, FI-O1621, Vantaa, Finland).
Predictive Factors
The baseline characteristics of 100 patients treated with TACE were evaluated. We analyzed the clinical and biological factors that may predict early recurrence of the disease: age, gender, viral markers (hepatitis B virus surface antigen and hepatitis C virus antibody), Child-Pugh score, serum AFP, HGF serum level, size and number of tumors).
Statistical Analysis:
· Statistical package for social sciences (IBM-SPSS), version 25 (IBM-Corporation, Chicago, USA; August 2017) was used for statistical data analysis.
· Data was expressed as mean, standard deviation (SD), number and percentage. Mean and standard deviation were used as descriptive value for quantitative data.
· Student t test was used to compare the means between two groups, and one-way analysis of variance (ANOVA) test was used to compare means of more than two groups. Mann-Whitney and Kruskal-Wallis tests were used instead of student t test and ANOVA for non parametric data to compare medians rather than means between two or more groups, respectively.
· Pearson Chi-square test was used to compare percentages of qualitative variables.
· Receiver Operating Characteristics curve (ROC curve) analysis was done to assess the predictive value of HGF as a screening test and to calculate the most suitable cut-off values which provide the highest possible accuracy (highest sensitivity and specificity at eh same time).
· Univariate binary logistic regression analysis was done to assess the possible risk factors for and those with significant univariate regression were included in multivariate binary logistic regression analysis model to estimate if any of them could be considered as an independent risk factor.
· For all these tests, the level of significance (p-value) was explained as:
² No significance p > 0.05;
² Significance p < 0.05;
² High significance p < 0.001.
3. Results
Comparison between the two groups: group (1) non early recurrence (NER) and group (2) early recurrence (ER), showed no significant difference in terms of age, sex, size of the tumor, aetiology of liver cirrhosis and liver function but AFP level, number of the lesions (multinodularity) and HGF level were significantly higher in the ER group compared with the other group (p value < 0.001, 0.001, 0.001) (Table 1).
Regarding HGF level there was a highly significant difference between the cases and the control group and the cut-off value of HGF was 1568.4 pg/ml as shown in Figure 1
There was a highly significant difference between in HGF level between the two groups as shown in Figure 2 and the cut-off value was 3960.4 pg/ml with a sensitivity of 97%, and a specificity of 100%.
Univariate binary logistic regression analysis for the possible risk factors of recurrence showed that AFP, multinodularity and HGF level were significant as shown in Table 2 (p value < 0.001, 0.001, 0.001).
Multivariate regression analysis showed that all of the AFP, multinodularity and HGF were inter-related and none of them could be considered as an independent risk factor as shown in Table 3.
![]()
Table 1. Demographic features and laboratory data of the participants studied.
HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NBNC: Non B Non C; T.bilirubin: Total bilirubin; PC: Prothrombin concentration; MWT: Mann Whitney test.
![]()
Table 2. Univariate binary logistic regression analysis for the possible risk factors of group (2) compared to group (1).
![]()
Table 3. Multivariate binary logistic regression analysis for the possible risk factors of group (2) compared to group (1).
![]()
Figure 1. ROC curve statistics for the predictive value of HGF to differentiate cases from controls.
ROC analysis shows that HGF can differentiate cases (groups 1 and 2 collectively) from controls, with a highly significant difference. Using coordinate points of the above ROC curve, the most relevant level cut-off value of HGF to differentiate cases from controls was 1568.4, with a sensitivity of 97%, and a specificity of 100%.
![]()
Figure 2. ROC curve statistics for the predictive value of HGF to differentiate group (2) from group (1).
ROC analysis shows that HGF can differentiate group (2) from group (1), with a highly significant difference. Using coordinate points of the above ROC curve, the most relevant level cut-off value of HGF to differentiate group (2) from group (1) was 3960.4, with a sensitivity of 88.9%, and a specificity of 96.4%.
- Triphasic CT images of Case (1) showed enhanced hepatic mass involving segment 8 in arterial phase (a) and wash out in delayed phase (b).
Digital subtraction angiography of the hepatic mass lesion was showed (c) and the angiography during Chemoembolization (d).
Disappearance of tumor vascularity and Lipiodol uptake as showed (e).
The one month post TACE follow up showed no residual viable tumor tissue (f).
- Triphasic CT images of Case (2) showed hepatic mass involving Lt hepatic lobe segment 3 in arterial phases (a).
Angiogaraphic blush of hepatic mass lesion was showed (b) and (c).
Follow up CT scan at the same level one month after TACE, showing marked response to treatment. (d) CT scan at the same level six months after TACE, showing reduced size with no residual enhancing tumor tissue. (e) CT scan at the same level one year after TACE, showing vanishing of the focal lesion with no residual enhancing tumor tissue (f).
- Triphasic CT images of Case (3) showed hypovascular mass lesion involving the right lobe (a) and the selective hepatic anigoraphy showing displacement of the hepatic vessels by the hypovascular tumor (b).
The procedure using microcatheter was shown (c) but Follow up CT showing poor response to chemoembolization (d).
4. Discussion
HCC is one of the most common cancers worldwide, and is associated with a high mortality rate. TACE is the most commonly used treatment modality when curative treatment is not indicated, we evaluated the predictive factors of early recurrence within 1 year in patients treated with TACE as a first therapy.
Multinodularity, elevated AFP and high HGF level were predictive factors of early recurrence. In respect to multinodularity, it is hypothesized that TACE cannot target undetected satellite lesions outside the zone of TACE induced necrosis. Consistent with the present study, Lee et al. [14] found that recurrence following initial remission by TACE has been more often reported in patients with multinodular HCC. By contrast Matsuda et al. [15] found that tumor multinodularity was not associated with 1-year HCC recurrence. This discrepancy may be explained in part by the different baseline characteristics of the patients with HCC between studies.
Triphasic CT can not detect microscopic remenant tumors during the early phase after TACE (4) Thus, HCC patients with multinodular lesions should be closely monitored even if they are in remission radiologically after TACE.
HCC with high AFP shows a poorer grade of differentiation than does HCC with lower AFP level [16]. Serum AFP has roles in immune system inhibition by altering the ratio of CD4+ and CD8+ T lymphocytes subsets and inducing lymphocyte death. Hypofunction of the immune system is an important factor for cancer cell growth, recurrence and metastasis. Although 10% to 30% of HCC cases are negative for AFP expression, studies have suggested that AFP is a significant predictor of HCC recurrence [17].
In our study, the serum HGF levels were 239 - 1260, 456 - 37,543, and 2654 - 38,790 in the control, group (A), and group (B), respectively. HGF was highly significantly elevated in the group (B) [Mean ± SD 4196 ± 5719 pg/ml] compared with that of the group (A) [5800 ± 5095 pg/ml] (p < 0.001).
Several researchers have confirmed similar results of increased HGF levels in patients with HCC compared with normal control participants such as the studies of Shiota et al. [18].
The sensitivity and specificity of HGF have been shown to vary with the different cut-off values used. According to our results, at a cut-off 1568.4 pg/ml (1.5684 ng/ml), with a sensitivity of 97%, and a specificity of 100%., and area under the curve (AUC) was 0.988. Yamagamim et al. [19] found in their study that the cut-off value of HGF for suspecting HCC in their cirrhotic patients was above 0.6 ng/ml and they concluded from their study that HGF may be a critical marker for emergence of HCC in their patients, they detected the HGF after only two days of storage of their samples, which is why their values were low and more specific (19). In our study, our samples were stored till the end of collection; this explains the higher cut-off value.
We conclude from this study that both HGF and AFP can be additional useful factors as noninvasive biomarkers for the early detection of HCC in cirrhotic patients and this agree with that was found by S. El Deen and El Haddad [20].
The use of HGF as an additional biomarker for early detection of HCC may be of value in cases with normal AFP and in other cases with persistent elevation in AFP that is associated with regeneration as a consequence of liver necroinflammation.
HGF is also involved in cancer invasion and metastasis as it is the most potent growth factor for hepatocytes and its receptor, the N-methyl-N’-nitroso-guanidine human osteosarcoma transforming gene transmembrane tyrosine kinase (c-Met) is implicated in HCC carcinogenesis and progression through activation of multiple signaling pathways that direct cell growth, proliferation survival, and motility [21].
Aberrant Met/HGF activation has been observed in many tumor types, can occur by multiple mechanisms, and promotes cellular proliferation and metastasis via growth factor receptors and other oncogenic receptor pathways.
On the contrary Karabulut et al. [22] found that HGF had diagnostic value but of no prognostic value in HCC patients.
The mechanism whereby stromal cells over-produce HGF for accelerating tumor malignancy has been investigated, and HGF-inducers (such as autacoids and cytokines) have been identified. Growing evidence indicates that NK4 inhibits tumor growth, invasion and metastasis in rodents bearing various types of malignant tumors and the discovery of NK4 as an HGF antagonist has promoted research in cancer biology, pathology and therapy [23].
Another mechanism by which HGF can promote tumor growth was highlighted by the studies which suggested that HGF increase the expression of VEGF, thereby initiating angiogenesis in a paracrine manner [24], HGF is a heparin-binding glycoprotein of mesenchymal origin that acts as a potent mitogen and motogen leading to organogenesis and tissue regeneration [25].
In conclusion, we have found that high AFP, multinodularity and high HGF were inter-related possible risk factors for 1-year recurrence of HCC in patients with initial remission following TACE. So, these parameters may be used to predict recurrence of HCC post TACE.
However, we should acknowledge that the present study has some limitations. This study included only patients candidate for TACE, several radiologists suggested that MRI should be used over CT for detection of viable tumor residuals after lipiodol based TACE. The 1-year follow up of recurrence may be short and need to be prolonged. So we recommend further studies to include patient candidates for locoregional therapy or surgery, the use of MRI for better follow up and extended duration of follow up more than 1 year.
Further studies with high sample sizes are needed to determine the potential clinical significance of these predictors and should include patients with early stages of the disease who are candidate for curative treatment modalities with longer period of follow up.
Appendix 1: Case (1)
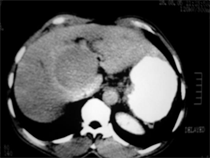
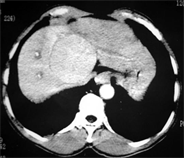
(a) (b)
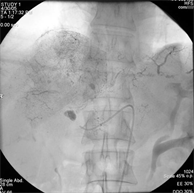
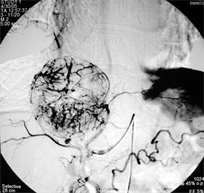
(c) (d)
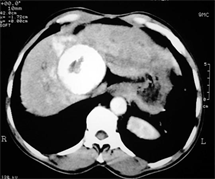
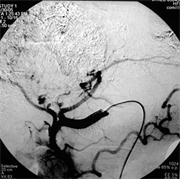
(e) (f)
Case (1). Triphasic CT scan of the liver showing hepatic mass involving segment 8 in arterial (a) and delayed phases (b). (c) Digital subtraction angiography showing hepatic mass lesion (d) Angiography during chemoeblization. (e) Digital subtraction angiography post TACE showing disappearance of tumor vascularity and Lipiodol uptake. (f) CT scan at the same level one month after the last TACE, showing marked response no residual viable tumors tissue.
Appendix 2: Case (2)

(a)
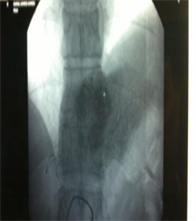
(b)
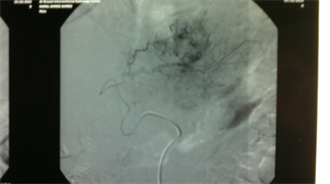
(c)
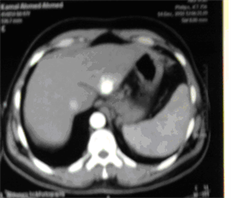
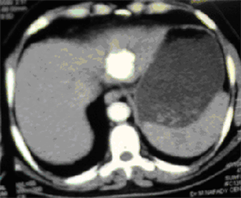
(d) (e)
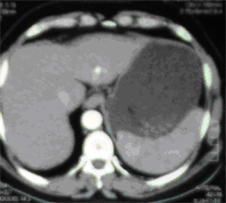
(f)
Case (2). (a) Triphasic CT scan of the liver showing hepatic mass involving Lt hepatic lobe segment 3 in arterial phases. (b) and (c): Digital subtraction angiography showing angiogaraphic blush of hepatic mass lesion. CT scan at the same level one month after TACE, showing marked response to treatment. (d) CT scan at the same level six monthes after TACE, showing reduced size with no residual enhancing tumor tissue. (e) CT scan at the same level one year after TACE, showing vanishing of the focal lesion with no residual enhancing tumor tissue (f).
Appendix 3: Case (3)
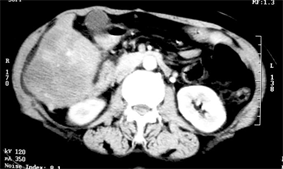
(a)
![]()
(b)
![]()
(c)
![]()
(d)
Case (3). (a) Arterial phase Triphasic CT scan of the liver showing hypovascular mass lesion involving the right lobe. (b) Selective hepatic anigoraphy showing displacement of the hepatic vessels by the hypovascular tumor. (c) The procedure using microcatheter. (d) Follow up CT showing poor response to chemoembolization.