Salinization Processes in the Benichab Coastal Aquifer-Mauritania ()
1. Introduction
Groundwater salinization is one of the main issues threatening the sustainable groundwater development in coastal regions. Its occurrence in many coastal aquifers shows a complex pattern, resulting not only from current processes but also from past events, including sea-level fluctuation, subsidence, climate change, mixing and human activity [1]. Different origins of groundwater salinization have been suggested through intensive research during the recent years: 1) actual seawater intrusion due to intense aquifer exploitation and/or sea level rise [2] [3] [4]; 2) mobilization of saline paleowaters due to past transgressions [5] [6]; 3) water-rock interaction like evaporite dissolution [7] [8]; 4) upcoming of saltwater from deep and adjacent reservoirs [9] [10] [11]; 5) anthropogenic contamination like return flow [12] [13] [14] [15] and most often; 6) a combination of some of these processes [10] [16].
Benichab region is under arid climate located in the coastal western part of the Mauritanian sedimentary basin. The region which is experienced a growing population and socio-economic development due to mining activities is facing an increasing water demand for domestic, pastoral, mining and socioeconomic development for this unique groundwater resource. Therefore, groundwater is being overexploited for drinking water and mining activities for this none renewable freshwater lense being affected by high salinity along its coastal fringeand relic of brine to brackish water identified in some deep boreholes whose origin has never been investigated. Works carried out in this system besides that made by Tasiast gold mine which data are used here are limited mainly to geophysical investigations for boreholes implementation.
The main objective of this paper is to characterize the geochemical processes associated to groundwater salinization in Benichab aquifer. Therefore, through the determination of major and minor ions and isotopic contents, we intend specifically to: 1) characterize the salinity distribution of groundwater 2) to identify the mechanism(s) of groundwater salinization.
Salinization processes can be diagnosed using many techniques such as geochemical and/or isotopic tools. As the useful indicators, Cl/Br molar ratio and stable isotope have been widely used to demonstrate the origin and transport processes of solutes in groundwater [17] [18]. Usually, major and minor elements concentrations such as Sr and Br can be used to identify origins of aquifer water salinization due to single process [2] [19]. Chloride concentrations and Cl/Br ratios are used to determine the sources of salinity, especially when seawater intrusion occurs [20]. When groundwater salinization results from a combination of several processes, environmental stable isotopes (δ18O and δ2H) are more efficient to identify origins of saline groundwater [21].
2. Study Area
2.1. Description of the Study Area
The study area is located between 18˚ and 20˚N, 15˚ and 16˚W, covering the NW part of Mauritania and an area of approximately 7900 km2. Benichab is the main city in the study area, and it is located in the south of the big city of Nouadhibou (Figure 1).
The climate in north-western Mauritania is arid with high evaporation rate and low rainfall amount. Nevertheless, some regional difference exists, with the highest mean annual rainfall (87 m) in the southern part of the area and lowest rainfall in the northern part (25 mm). With such a low rainfall amount and high potential evaporation of nearly 3700 mm/year, the groundwater recharge can only occur at favorable sites. The temperature ranges from 28˚C in January to 42˚C in June.
The topography of the study area is characterized by moderate relief composed mostly of Ogolian dunes (20,000 to 12,000 years BP) separated by inter-dune channels known locally as “gouds”.
The economic activities of the population are based on extensive livestock farming, traditional sea fishing and gold artisanal exploitation. Also, mining companies occupy an important place in the economy of this region with Kinross (gold) and MCM (copper and gold) establishment since 2017.
2.2. Geology and Hydrogeology Framework
The Benichab region belongs to the large Senegal-Mauritanian basin. The local geology consists of a succession of sedimentary series of marine, fluvial and aeolian origin, from the Meso-Cenozoic to Quaternary age [22] [23] [24] [25]. These sedimentary formations cover the basement rock that outcrops in the eastern and northern part of the region. During the Quaternary, the region experienced several marine transgressions. The most recent was the transgressive period of the Nouakchottian [26], which may be the source of the groundwater
![]()
Figure 1. Location of the study area and sampling network.
salinity. A geological cross section established using the hydraulic boreholes data shows the configuration of the aquifer in the study area (Figure 2) made of Continental Terminal (CT) formations which are detritic materials with discontinuous interbedded sandstone, sandy clay, clayey sand, sandy lenses and clay. They constitute an unconfined aquifer which sediments are heterogeneous both laterally and vertically. The groundwater level monitoring since 2015 show no significant seasonal variation and flow direction is mainly from the northwest to the southeast precisely from the Atlantic Ocean to the continent.
3. Sampling Analysis
Groundwater was collected from 45 sampling points composed of dug-wells and boreholes during 4 campaigns (wet and dry season) from 2015 to 2017 (Figure 3). Water table depth, Temperature, pH, electric conductivity and salinity were measured using a portable meter (WTW device) in the field. Water samples were taken after purging the wells until constant value of conductivity obtained. Groundwater samples for chemical analyses were filtered and samples for cation analyses were acidified to pH < 2 with HCl. All collected samples were stored at 5˚C until analyses. Major and minor elements were analyzed at the Al control Laboratory in UK through various procedures: ICP-MS for Ca, Na, Mg, Fe, K, B and Sr; spectrophotometry for SO4, Cl and NO3; chromatography for Br. The analysis of the stable isotopes was carried out at the Sfax (Tunisia) and Avignon (France) laboratories. Results were expressed relative to the international standards (V-SMOW for δ18O and δ2H) in ‰. The analytical uncertainties were ±0.3‰ δ18O and ±2‰ for δ2H.
4. Results
4.1. Salinity Distribution and Water Type
Statistics of physico-chemical parameters and chemical composition of 119
![]()
Figure 3. Piezometric map and EC distribution (mean value of all campaigns).
groundwater samples through 4 field campaigns during 2015-2017 are shown in Table 1. The water samples exhibit pH values circumneutral to basic (6.8 to 8.6) and TDS values calculated from EC ranging from 140 to 38,613 mg/L. The distribution of EC values shows a gradual increase from fresh groundwater lense (EC value < 1 mS/cm) towards the Atlantic Ocean where the groundwater is highly saline (EC ranging from 25 to 45.5 mS/cm). The most saline waters are observed in wells located in the north near the coastal area. This gradual pattern is evidenced by the groundwater flow mainly from the ocean to the aquifer (Figure 3) suggesting potential seawater intrusion.
Chemical data plotted to the Piper diagram [27] show different water types spatially distributed (Figure 4):
1) m-HCO3 and Na-HCO3, representing the fresh Groundwater lense;
2) Na-Cl occurring in the coastal area represents the saline waters;
3) Na-m, m-Cl and mixed facies, a mixture type between fresh water and saline water. The gradation can be seen from Na-Cl (saline water) and m-HCO3 (fresh water), particularly in the cation triangle where the alignment is linear.
4.2. Hydrogeochemical Process
4.2.1. Geochemical Process
It is known that water chemistry depends on mixing of different water bodies and/or geochemical reactions such as dissolution-precipitation, evaporation, ion exchange processes. In order to discriminate about processes driving the chemical composition of groundwater, bivariate diagrams (ions/Cl vs Cl) are useful tools (Figure 5) to identify mixing mechanisms of fresh and saline groundwater from additional chemical reactions [2].
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 4. Water types [(a): Piper diagram of all samples; (b) Count plot of facies abundance; (c) Spatial distribution of facies (mean value of all campaigns)].
![]()
Figure 5. Bivariate plots of major ions relative to Cl vs Cl [(a) Na/Cl vs Cl; (b) Ca/Cl vs Cl; (c) K/Cl vs Cl; (d) Mg/Cl vs Cl; (e) SO4/Cl vs Cl; (f) HCO3/Cl vs Cl)]. The legend is the same as in Figure 4.
In the lowest salinity group (Na-HCO3, m-HCO3, Na-m), the freshwater has high molar ratio while the saline group (Na-Cl) has molar ratio that are close or even below the value of sea water which is the main source of salinization. However, Na+, K+, Mg2+, Ca2+ and SO2− 4 relative to chloride molar ratios increase suggest some contributions of other processes occurring in the fresh water group such as hydrolysis of silicate minerals, ions exchange reaction, gypsum dissolution, calcite dissolution [28]. The relationship between HCO3/Cl ratios and Cl also exhibits a pronounced negative slope indicating the reverse relationship between carbonate (freshening) and Cl (salinization) [2] [29].
![]()
Table 1. Statistics values of physico-chemical parameters and chemical analysis.
Based on the inverse of Simpson’s ratio as reported by [30], the relative abundance of saline water can be itemized into six classes (Figure 5(f)). In general, a HCO3/Cl ratio > 2 indicates freshwater whereas a ratio less than unity indicates higher degrees (or potential) of salinization [31]. Fresh waters include Na-HCO3 and m-HCO3 facies, slightly/moderate saline waters are Na-m and m-m facies and saline waters are Na-Cl and m-Cl. Severely saline waters are of Na-Cl facies mostly located in the northern part of the study area.
To investigate the other potential sources of groundwater mineralization, different correlations were established between ions (Figure 6). Figure 6(a) shows in one hand, a positive correlation between Ca + Mg and HCO3 + SO4 close to [1:1] equilibrium line in the fresh water group indicating that the carbonate and sulphate minerals dissolution are involved in groundwater mineralization. In the other hand, the majority of saline samples are above the equilibrium line [1:1], which indicates the presence of inverse ionic exchange under seawater intrusion influence [32] [33]. This latter process is exhibited in the graph [(Ca + Mg) − (HCO3 + SO4)] vs [(Na + K) − Cl] (Figure 6(b)) by linear negative trend [34] [35].
In general, the increase in the concentration of Sr parallel to that of Cl ion is largely due to the saltwater. However, in water-rock interaction system, this process is not the only source of Sr in aqueous phase. A poor correlation between Sr and Cl in the saline groundwater group indicates an additional input of
![]()
Figure 6. Bivariate plots exhibiting silicate weathering and ionic exchange [(a) Ca + Mg vs HCO3 + SO4; (b) (Ca + Mg) − (HCO3 + SO4) vs (Na + K) − Cl; (c) Sr vs Cl; (d) Sr vs Ca]. The legend is the same as in Figure 4.
Sr [28]. Plots of Ca vs Sr (Figure 6(c)) show a linear correlation which indicates Sr input from carbonate dissolution or precipitation and gypsum. The mean Ca/Sr molar ratios in groundwaters (141) exceed the seawater value (113) with highest values found in the fresh groundwater group (278). The lowest values found in the saline water group indicate concomitant reverse ion exchange where Ca is released and Na absorbed at clay minerals surface.
4.2.2. Salinization
Minor elements, such as Br and B can be valuable markers of fresh/seawater mixing. The Br/Cl ratios have been used to distinguish salinity of marine and non-marine origins [36]. Because Br and Cl are relatively conservative in hydrological systems, the Br/Cl ratios of fresh waters are primarily controlled by the initial ratio in precipitation. As sea salt spray is the primary source of these ions, precipitation should have a Br/Cl ratio similar to that of seawater. Thus, the Br/Cl ratio should be constant when salinization of fresh water occurs by simple mixing of seawater if there is no anthropogenic contamination [2].
On the bivariate plot of Br/Cl ratios expressed in [1000*Br/CL] vs Cl (Figure 7(a)), as Cl concentration increases, the Br/Cl ratios of saline groundwaters are maintained in the normal seawater range (0.7 to 2.0), indicating that the
![]() (a)
(a) ![]() (b)
(b)
Figure 7. Bivariate plots of minor elements relative to Cl vs Cl [(a) Br*1000/Cl vs Cl; (b) B*1000/Cl vs Cl]. The legend is the same as in Figure 4.
salinization of these groundwaters results mainly from mixing precisely with seawater. The lowest Br/Cl ratio (0.07) suggests a potential source of Br, probably anthropogenic, other than seawater as evidenced by [37]. Moreover, B/Cl ratios of all saline groundwaters are similar to the seawater ratio, suggesting that their salinities mainly result from seawater.
4.2.3. Quantification of Seawater Intrusion
The fraction of seawater (fsw) was calculated from the Cl concentration. This latter is considered as conservative tracer not participating in the ion exchange process [38]. The ratio is calculated using Equation (1) [39]:
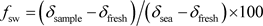 (1)
(1)
With δsample as sample chloride concentration; δfresh as freshwater chloride concentration and δsea as seawater chloride concentration. The two end members of the mass balance equation represent the mean composition of the fresh groundwater adjacent to the saline front and the seawater composition, respectively.
The computed values of seawater contribution vary from 0% to 100% with a mean value of 8.42%. As already classified with the Piper diagram, saline waters are represented mostly by Na-Cl facies (Figure 8(a)). High mixing rates in Na-Cl group in the box-plot (Figure 8(b)) are found in wells located in the northern part of the study area where Cl content can exceed the value of sea water due to high evaporation uptake. This is in agreement with groundwater flow direction which indicate inflow from the ocean towards the aquifer and the positive trend between Cl and Br (R2 = 0.9) suggesting marine source.
Beside the evidence of actual inflow from the ocean, sea level in coastal Benichab has fluctuated by approximately 1 - 3 m around its current elevation over the last six millennia (post-6000 Holocene) [25]. Therefore, the study area was regularly submerged by seawater. During the regression phases, part of the seawater was trapped inside the detrital deposits, and the different deposits have incorporated a significant amount of salts [40] [41]. In addition to this, the high
![]() (a)
(a) ![]() (b)
(b)
Figure 8. Seawater mixing [(a) Box plot water type vs seawater ratio; (b) Spatial distribution of seawater ratio (mean values of all campaigns)].
evaporation rate associated to the aridity of the study area and the low hydraulic gradients of the aquifer has produced these paleo-saline waters observed in the Northern part of Benichab (Figure 8(b)).
4.3. Stable Isotope Geochemistry
The stable isotopes of oxygen and hydrogen are generally considered to be transported conservatively in shallow aquifer settings, prevalence of high water-rock ratios and an absence of significant evaporation. Nevertheless, in the Benichab where temperature can reach 42˚C (in June) as well as high evaporation (3700 mm/year), it is likely that rainfall evaporates before and even after infiltration had occurred. Therefore, the values of the isotopic signature should be combined with the other finger-prints to evaluate the salinization processes [28].
The GNIP data from Louga station (Senegal), which is the closest to the study area, were used for isotopic characterization of precipitation. They range from −6.0‰ to −1.9‰ with a mean of −3.8‰ for δ18O; and −42.69‰ to −8.30‰ with a mean of −25.04‰ for δ2H. According to the Ordinary Least Square Regression (OLSR) method proposed by [42], the Local Meteoric Water Line (LMWL) defined by δ2H = (7.38 ± 0.90)*δ18O + (3.02 ± 1.00) indicates values quite different from that of GMWL [43]; suggesting the occurrence of fractionation processes, particularly evaporation.
Isotopic compositions of the groundwater samples range from −1.8‰ to −6.9‰ for δ18O with a mean of −5.0‰; from −23.72‰ to −47.6‰ for δ2H with a mean value of −41.44‰. The lower mean values of δ18O and δ2H as compared to those of mean rainfall values indicate ancient water occurring in the system. This is evidenced by no tritium occurrence in all groundwater samples. Despite that, all samples cluster below the LMWL, evidencing enrichment in their stable isotope compositions with a slope of 5 (Figure 9(a)). Such a low slope can be produced by evaporation and/or mixing of groundwater and seawater [2]. In this
![]() (a)
(a) ![]() (b)
(b)
Figure 9. Stable isotopes [(a) δ2H vs δ18O; (b) Cl vs δ18O].
case, the combination of these two processes seems occurring because of the curve trend revealed by the Cl-δ18O relationship (Figure 9(b)).
5. Conclusions
The main purpose of this study was to examine the salinization of groundwater in coastal area and to identify the main processes driving the groundwater mineralization. Physco-chemical parameters and isotopic contents were examined as multiple lines of evidence for geochemistry understanding of the Benichab aquifer. 1) The groundwater flow direction indicates inflow mainly from the northwest to the southeast precisely from the Atlantic Ocean toward the aquifer. This mechanism is characterized by gradual decreases in conductivity values which range from 45,200 µs/cm to 227 µs/cm. 2) The hydrochemical characteristics of groundwater comprise three main types: a) fresh-groundwater, free of marine water constitutes of m-HCO3 and Na-HCO3 facies located in the far Est of the study area with an EC < 1mS/cm; b) saline groundwater mainly of Na-Cl facies occurring in the coastal area identified by high EC values (5 to 45.2 mS/cm) and Cl contents; and c) brackish water represented by Na-m, m-Cl and mixed facies which is a mixture type between fresh water and saline water. 3) Na/Cl and Br/Cl ratios in saline water suggest seawater intrusion which is the main source of salinity. Seawater mixing ratios vary from 0% to 100% with a mean value of 8.42%. The highest ratios are founded in the northern part of the study area where Cl content can exceed the value of sea water. Due to high evaporation uptake, the variation in chemical composition of groundwater demonstrates other hydrogeochemical processes, such as mineral weathering and cation exchange that are more noticeable in the freshwater group. 4) The stable isotopic composition of groundwater (δ18O and δ2H) to Chloride (Cl-δ18O) indicates other process (mainly evaporation) to occur rather than a simple mixing of fresh groundwater with seawater. Despite these valuable results obtained through this study, further works should be carried out to infer likely recharge processes and impact of pumping in the dynamic of the system and seawater advance as well as age dating using 3H and 14C.
Acknowledgements
The authors gratefully acknowledge Tasiast Mauritanie Limited S.A. for its financial support and the anonymous reviewers for their constructive comments and suggestions.