The crystal structure and the dynamic feature of molecular structure in solution for 1,8-dibenzoyl-2,7-dimethoxynaph-thalene are revealed by X-ray crystallographic analysis and VT-NMR measurements. In crystal, the molecule of the title compound is located on a twofold rotation axis. The two benzoyl groups are situated in an opposite direction. The dihedral angle between the mean planes of the phenyl ring and the naphthalene ring system is 80.25(6)

. The benzene ring and carbonyl moiety in each benzoyl group are almost coplanar. The molecular packing is stabilized by weak C–H…O hydrogen bonds and aπ-πstacking interaction between the benzene rings [centroid-centroid and interplanar distances of 3.6383(10) and 3.294
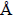
, respectively]. In solution, the temperature-dependent rotation behavior of the C–C bond between the benzene ring and the ketonic carbonyl group has been observed by1H VT-NMR measurements. Furthermore, comparison of the C–C bond rotation behavior between the benzene ring and the carbonyl group with 1-ben-zoyl-2,7-dimethoxynaphthalene has clarified that the C–C bond between the ketonic carbonyl group and the naphthalene ring rotates slower than the 1,8-dibenzoylated homologue.