Studies and Mechanism of Olefination Reaction in Aryl-Enolates with Paraformaldehyde ()
1. Introduction
The α-substituted acrylic acid analogs or derivatives are a dynamic key synthon in the construction of interesting molecules due to their capacity to act as Michael acceptors [1] [2] [3] [4] [5] , Diels-Alder dienes [6] or Aza-Morita-Baylis-Hillman reaction substrates [7] . These molecules include drugs, bioactive compounds, process impurities and advanced synthetic intermediates [8] [9] . As a result, several methods to synthesize these synthons have been reported (Figure 1) [10] [11] .
One of the most common choices of aldehyde for α-substituted-α,β-unsaturated compound through aldol condensation is formaldehyde where the reaction is typically known as α-methylenation [12] [13] [14] .
Excellent works about the use of aqueous formaldehyde as methylenation agent via Mannich reaction have been published. For example, in 2006, Erkkilä and Pihko have performed the formation of α-methylenated aldehydes, also
![]()
Figure 1. Strategies for the synthesis of acrylates.
called α-substituted acroleines, using aqueous formaldehyde and secondary amines as a catalyst [12] . Several studies employing other aldehydes and different types of alkyl, cyclic, aryl ketones and esters have been developed using diisopropylammonium trifluoroacetate salt [13] or Meldrum’s acid as a catalyst.
On the other hand, and in addition to Mannich reaction, one of the most useful strategies for the formation of α,β-unsaturated systems is the aldol condensation [15] [16] due to its efficiency and low cost. In this transformation, the α-carbon of an enolate is bonded with the carbonyl carbon of an aldehyde, and a β-hydroxylated-carbonyl intermediate is obtained. Most of the times, a β-dehydration is observed as part of the process, and an α,β-unsaturated compound is isolated. On the other hand, when a functionalized aldehyde is employed, a trans-β-substituted-α,β-unsaturated compound is obtained [17] [18] . Rodriguez et al. [19] reported that when both enolate and aldehyde are functionalized, the corresponding product is an α,β-disubstituted-α,β-unsaturated compound. They also mentioned that with this method, stereochemistry control of this reaction proved to be nontrivial.
Even though the aldol reaction employing formaldehyde is useful in the formation of α-methylenated carbonyl compounds, there are few reports about its use. One of the early reports was that of Laos in 1967 [20] where the α-methylenation of steroidal ketones was carried out with aqueous formaldehyde and potassium acetate as base and methanol or water as solvents. Recently, Liu studied the effect of the acidity of zeolite in the formation of acrylic acid and methyl acrylate from formaldehyde and methyl acetate [21] .
A useful and practical source of formaldehyde is paraformaldehyde, a polymer, due to it is a versatile and easily handled reactively. For example, Amri et al. [22] used paraformaldehyde as homologate agent by substituting phosphonate group in the Honer-Wadsworth-Emmons reaction type synthesis of (±)-homosarkomycin with 98% of yield. An aldol type α-methylenation of lactones employing paraformaldehyde, which gives moderate to good yields was performed by Tanaka and Yamashita [23] . Chen et al. [24] also prepared α-nitro ethyl acrylate intermediates using paraformaldehyde, which was then employed as Michael acceptor in the synthesis of tryptophan derivatives.
Traditionally, it is accepted that aqueous formaldehyde and paraformaldehyde are two different sources of the same monomeric reactive and that the only advantage that paraformaldehyde has is that it can be used in water free reactions or solvents.
In the present work, we propose a possible mechanistic pathway of aldol condensation using paraformaldehyde, which is different from that observed in formaldehyde.
2. Results and Discussion
2.1. Reaction of Methyl Phenylacetate (1a) with Sodium Hydride and Paraformaldehyde (3)
Currently, our research group is interested in the synthesis of β2-and β3-amino acids via aza-Michael addition to α,β-unsaturated esters [25] . One of our method of choice is a facile synthesis of 2-aryl methyl acrylates (4a-d); retrosynthetic approximation is shown in Scheme 1.
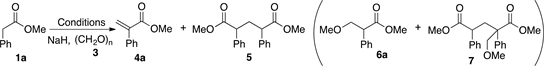
In the present study, initially, methyl phenylacetate (1a), paraformaldehyde (3) and NaH were chosen as starting materials. Acrylate 4a synthesis was carried out using toluene as solvent and microwave (MW) heating. Table 1 and Scheme 2 summarize experimental results for different solvents, reaction times, and sources of activation energy.
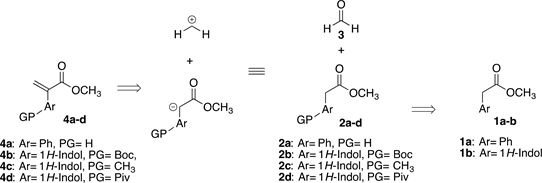
Scheme 1. Retrosynthetic analysis for the preparation of 2-aryl methyl acrylates.
![]()
Table 1. Reaction conditions and yields for the aldol condensation of 1a and 3.
a. Traces of product 4a, b. Temperature of addition of the reagents.
Scheme 2. Aldol condensation reaction of 1a and 3.
The first entry in Table 1 shows the result of the formation of the acrylate 4a using 1.5 equiv. of NaH in toluene and MW heating for 1 h. Compound 5 was isolated as the main product with 55% yield and only traces of 4a (entry 1). An increase of paraformaldehyde from 3 to 9 equiv. and of NaH from 1.5 to 3 equiv., results in a better yield (60%) of acrylate 4a (entry 2). On the other hand, when the reaction is carried out with 16.6 mmol of 1a, acrylate 4a was isolated in higher yield (87% yield, entry 3). When the reaction is carried out at room temperature, 4a was obtained with a smaller yield and 6a, and 7 were also produced in 29% and 5% yields, respectively (entry 4). For this last experiment, it is relevant mentioning that after 1 h, the reaction spontaneously generated an exothermic from 25˚C to 55˚C.
When the reaction was carried out at 55˚C, after 1 h only 4a was obtained with 87% yield (entry 5). The result suggests that temperature can be used to control the production of byproducts 6a and 7a. Finally, in order to explore the solvent effect, the reaction was carried out in THF at the same temperature with a better yield of 4a in only 15 min (entry 6).
Scheme 3 proposes a reaction mechanism for the formation of 4a, 5, 6a, and 7. The enolate of 1a carried out a nucleophilic substitution over paraformaldehyde, and the intermediate 10 was produced.
From there on, the reaction could generate 4a through a β-elimination (Eβ, Path 1) and subsequently a Michael addition of the enolate of 1a on 4a to form the corresponding compound 5.
Alternatively, two products could be generated via Path 2. 6a is obtained through a nucleophilic substitution by the hydride; and 7 was isolated through the Michael addition of 4a and the enolate 6a.
Recrystallization of 7, afforded a suitable crystal for X-ray diffraction analysis. The resulting structure is presented in Figure 2.
2.2. Synthesis of Acrylates Derivatives 4b, 4c, and 4d
We chose to examine the synthesis of other acrylates: 4b, 4c, and 4d under the conditions of entry 6 in Table 1 which are optimized from the reaction time and solvent choice point of view. For this purpose, we first synthesized 2b, 2c, and 2d (see Scheme 4).
Due to the structural diversity presented in methyl esters 2b, 2c, and 2d, a general synthetic route to these compounds was not available. Below, we describe two methodologies, including some developed by our research group.
Compound 1b was first esterified with methanol in the presence of TMSCl. 2b and 2d were then synthesized through of the addition of (Boc)2O or pivaloyl
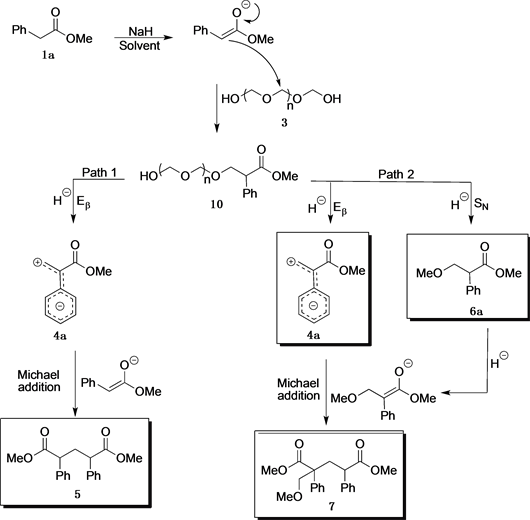
Scheme 3. Mechanism approach for the isolation of byproducts 5, 6a and 7.
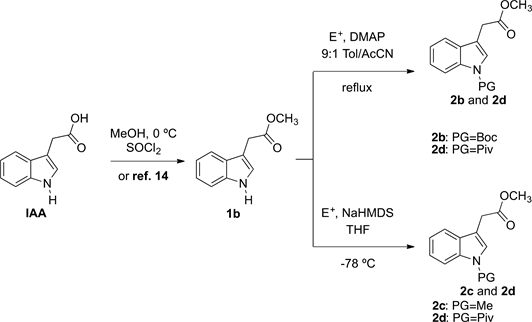
Scheme 4. Syntheses of derivatives 2b-d.
chloride, with 4-DMPA as a catalyst in a mixture of Toluene/AcCN 9:1 as solvent at reflux. On the other hand, 2d can also be produced by the addition of NaHMDS to 1b in THF at −78˚C.
Recrystallization of 2d afforded suitable crystal for X-ray diffraction analysis shown in Figure 3.
Having produced 2b, 2c, and 2d, we then proceed with the syntheses of the acrylates 4b, 4c, and 4d. The results of this series are summarized in Table 2.
As expected from the results shown in Table 1, the desired acrylates derivatives 4b and 4c were obtained in 50% and 62% yield respectively. After purification of 4b by column chromatography, traces of 6b were found (entries 1 and 2, Table 2 and Scheme 5). It is worth mentioning that the product 4d could not be obtained under these reaction conditions, but surprisingly one side product (8d) was isolated in 37% yield.
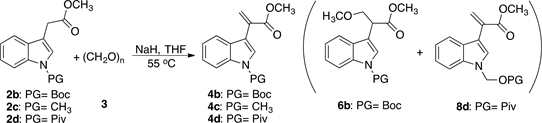
Scheme 5. Aldol condensation reaction of 2a-c and 3.
![]()
Table 2. Reaction conditions and yields for the aldol condensation of 2b-d with 3.
aTraces of product 6b.
Similar to the case of 2b, here we expect to obtain either 4d or 6d. Instead, we found 8d a product of rearrangement reaction. We hypothesize that this compound formed by the insertion of a -CH2O-moiety from paraformaldehyde between the protector group and the indole (Scheme 6).
3. Conclusion
In summary, although the classical mechanism for this olefination reaction suggests that the paraformaldehyde is dissociated to formaldehyde when it is warmed, and then it reacts with the enolate to get the aldol product and its subsequent dehydration. However, in this study, we demonstrate that the addition of a carbon atom from paraformaldehyde to give rise to the vinyl group occurs through a series of nucleophilic substitutions catalyzed by hydride over acetalic carbons from the polymer.
4. Experimental
Experimental Materials and Methods
The course of the reactions was followed by TLC. Silica gel of 70 - 230 mesh of Merk (Darmstad, Germany) was used for purification of the products by flash chromatography. Methyl phenyl-acetate (1a), 3-indoleacetic acid and paraformaldehyde (3) were purchased from Aldrich and used without further purification.
Analytical Methods
Spectra data of 1H NMR and 13C NMR were obtained in CDCl3 solutions with TMS as internal standard on Varian Gemini 200, Varian Oxford 400, and Inova 400 spectrometers. Mass spectral analyses were carried out in a spectrometer JEOL model JMS-AX50SHA.
Methyl 2-(1H-Indol-3-yl)Acetate 1b.
In a flask of 250 mL provided with magnetic stirrer, 5.0 g (28.54 mmol) of 3-indolacetic acid and 50 mL of MeOH were added. The flask was cooled at 0˚C
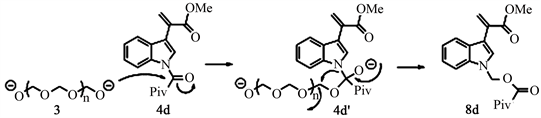
Scheme 6. A proposed mechanism for the formation of 8d.
and then 2.5 mL (4.08 g, 34.27 mmol, 1.2 equiv.) of thionyl chloride. The mixture was stirred during 1 h, and then a saturated K2CO3 solution was added until getting 8 - 9 pH. Methanol was evaporated, and the solution was extracted with ethyl acetate (3 × 30 mL). The organic phase was dried over Na2SO4 anhydrous. Concentration in a rotatory evaporator gave the crude product, which was purified by flash chromatography (n-hexane/ethyl acetate, 70:30 - 50:50). Red oil, yield 97%. 1H NMR (CDCl3, 400 MHz), δ (ppm): 3.72 (s, 3H, OCH3); 3.80 (d, 3J = 0.8 Hz, 2H, CH2CO); 7.04 (d, 3J = 2.4 Hz, H, NHCH); 7.18 (m, 2H, C5H-C6H); 7.29 (d, 3J = 8.4 Hz, H, C4H); 7.63 (d, 3J = 7.6 Hz, H, C7H); 8.16 (br, s, H, NH). 13C NMR (CDCl3, 100 MHz), δ (ppm): 31.1 (OCH3); 31.9 (CH2CO); 108.2 (C-3); 111.2 (C-4); 118.7 (C-7); 119.6 (C-5); 122.1 (C-6); 123.2 (C-2); 127.2 (Cipso-3a); 136.2 (Cipso-7a); 172.7 (CH2CO). HRMS (FAB+): calcd. for C11H11NO2[M+]: 189.2140, found: C11H12NO2[M + H+]: 190.0880.
General procedure 1:
In a flask provided with magnetic stirrer 1b (or c), 1.1 equiv. of (Boc)2O, 0.1 equiv. of 4-DMAP and a 9:1 Toluene: CH3CN mixture were added. The reaction mixture was refluxed for 3 h. The mixture of solvents was evaporated, and then H2O was added and extracted with ethyl acetate. The organic phase was dried over Na2SO4 anhydrous. Concentration in a rotatory evaporator gave the crude product, which was purified by flash chromatography (n-hexane/ethyl acetate).
General procedure 2:
In a flask provided with a magnetic stirrer and N2 atmosphere, 1c (or d) and 50 mL of THF anhydrous were added. The flask was cooled at −78˚C and then 1.1 equiv. of NaHMDS and 1.2 equiv. of protective reagent. The mixture was stirred during 2 h, and then a saturated K2CO3 solution was added until getting 8 - 9 pH. The solution was extracted with ethyl acetate. The organic phase was dried over Na2SO4 anhydrous. Concentration in a rotatory evaporator gave the crude product, which was purified by flash chromatography (n-hexane/ethyl acetate).
General procedure 3:
In a flask of 100 mL provided with magnetic stirrer 5 equiv. of NaH 60% were added to 50 mL of n-hexane. The mixture was stirred during 15 min, the n-hexane was subtracted, and 50 mL of THF were then added. The flask was cooled at 0˚C and then 2, 9 equiv. of paraformaldehyde were added. The reaction mixture was stirred for 1 h, 20 mL of water were added, and the solution was extracted with diethyl ether (3 × 10 mL). Concentration in a rotatory evaporator gave the crude product, which was purified by flash chromatography (n-hexane/ethyl acetate).
tert-Butyl 3-(2-Methoxy-2-Oxoethyl)-1H-Indole-1-Carboxylate 2b.
According to General Procedure 2, in a flask of 250 mL, 5.0 g (26.43 mmol) of 1b, 6.34 g (29.068 mmol) of (Boc)2O, 0.32 g (2.64 mmol) of 4-DMAP and 100 mL of a 9:1 Toluene:AcCN mixture were added. Green solid, yield 97%. m.p.: 58 ºC. 1H NMR (CDCl3, 200 MHz), δ (ppm): 1.96 (s, 9H, OC(CH3)3); 4.01 (s, 5H, CH2COOCH3); 7.58 (m, 2H, C5H-C6H); 7.82 (d, 3J = 8.0 Hz, H, C4H); 7.87 (s, H, NCH); 8.45 (d, 3J = 8.0 Hz, H, C7H). 13C NMR (CDCl3, 50 MHz), δ (ppm): 28.4 (OC(CH3)3); 31.1 (OCH3); 52.3 (CH2CO); 83.8 (OC(CH3)3); 113.2 (C-3); 115.4 (C-7); 119.1 (C-4); 122.7 (C-6); 124.5 (C-5); 124.6 (C-2); 130.1 (Cipso-3a); 135.5 (Cipso-7a); 149.6 (OCON); 171.5 (CH2CO). Anal. Calcd. for C16H19NO4: C 66.42, H 6.62; N, 4.84; found: C 66.10, H 6.49, N 4.66.
Methyl 2-(1-Methyl-1H-Indol-3-yl)Acetate 2c.
According to General Procedure 2, 0.48 g (2.54 mmol) of 1b, 2.8 mL (2.8 mmol) of NaHMDS and 0.19 mL (3.04 mmol) of iodomethane were added. Colorless oil, yield 29%. 1H NMR (CDCl3, 200 MHz), δ (ppm): 3.68 (s, 3H, NCH3); 3.73 (s, 3H, OCH3); 3.76 (s, 2H, CH2CO); 7.02 (s, H, NCH); 7.14 (m, 2H, C5H-C6H); 7.28 (d, 3J = 7.0 Hz, H, C4H); 7.58 (d, 3J = 10.0 Hz, H, C7H). 13C NMR (CDCl3, 50 MHz), δ (ppm): 31.2 (NCH3); 32.8 (OCH3); 52.0 (CH2CO); 105.1 (C-3); 106.9 (C-4); 109.4 (C-7); 119.0 (C-5); 119.3 (C-6); 121.9 (C-2); 127.8 (Cipso-7a); 137.0 (Cipso-3a); 172.7 (CH2CO). HRMS (FAB+): calcd. for C12H13NO2[M+]: 203.2410, found: C12H13NO2 [M+]: 203.0946.
Methyl 2-(1-Pivaloyl-1H-Indol-3-yl)Acetate 2d.
According to General Procedure 2, 0.5 g (2.64 mmol) of methyl 1b, 2.9 mL (2.9 mmol) of NaHMDS and 0.39 mL (3.17 mmol) of trimethylacetyl chloride were added. Colorless solid, yield 29%. m.p.: 108˚C - 111˚C. 1H NMR (CDCl3, 200 MHz), δ (ppm): 1.52 (s, 9H, (CH3)3CO); 3.74 (s, 3H, OCH3); 3.75 (d, 3J = 2.0 Hz, 2H, CH2CO); 7.32 (m, 2H, C5H-C6H); 7.52 (d, 3J = 8.0 Hz, H, C4H); 7.80 (s, H, NCH); 8.51 (d, 3J = 8.0 Hz, H, C7H). 13C NMR (CDCl3, 50 MHz), δ (ppm): 28.6 (OC(CH3)3); 30.7 (OCH3); 41.2 (OC(CH3)3); 52.1 (CH2CO); 104.9 (C-3); 113.8 (C-7); 117.4 (C-4); 118.4 (C-6); 123.5 (C-5); 124.2 (C-2); 129.0 (Cipso-3a); 136.9 (Cipso-7a); 171.3 ((CH3)3CO); 176.8 (CH2CO). HRMS (FAB+): calcd. for C16H19NO3 [M+]: 273.3320, found: C16H20NO3 [M + H+]: 274.1457. X-Ray crystallographic structure in Figure 2 [26] .
Methyl 2-Phenyl-Acrylate 4a.
In a flask of 250 mL provided with a magnetic stirrer and N2 atmosphere 4.0 g (100 mmol, 3 equiv.) of NaH 60% were added to 50 mL of n-hexane. The mixture was stirred during 15 min, the n-hexane was subtracted, and 125 mL of THF was then added. The flask was cooled at 0˚C, and then 5 g (33.31 mmol, 1 equiv.) of 2a, 9 g (299.9 mmol, 9 equiv.) of paraformaldehyde was added. The reaction mixture was heated to 50˚C - 53˚C for around 8 min, intense reflux was initiated, and the mixture immediately turns yellow. Then 50 mL of water was added, and the solution was extracted with ethyl acetate (3 × 20 mL). Concentration in a rotatory evaporator gave the crude product, which was purified by flash chromatography (n-hexane/ethyl acetate, 80:20 - 60:40). Colorless oil, yield 87%. 1H NMR (CDCl3, 400 MHz) δ (ppm): 3.81 (s, 3H, CH3O); 5.88 (d, J = 1.2 Hz, 1H, CHb-gem), 6.36 (d, J = 1.2, 1H, CHa-gem), 7.10 - 7.41 (m, 5H, Ph). 13C NMR (CDCl3, 100 MHz) δ 52.3 (CH3O), 126.9 (CH2=C), 128.2 (CPh), 128.3 (CPh), 128.4 (CPh), 136.8 (Cipso-Ph), 141.4 (C=CH2), 167.3 (COOCH3). HRMS (EI): calcd. for C10H10O2[M]+: 162.0681, found: C10H10O2: 162.0070.
tert-Butyl 3-(3-Methoxy-3-Oxoprop-1-en-2-yl)-1H-Indole-1-Carboxylate 4b.
According to General Procedure 3, 0.52 g (1.8 mmol) of 2b, 0.36 g (9 mmol) of NaH 60% and 0.486 g (16.18 mmol) of paraformaldehyde were added. Green oil, yield 50%. 1H NMR (CDCl3, 400 MHz), δ (ppm): 1.28 (s, 9H, OC(CH3)3); 3.35 (s, 3H, OCH3); 5.85 (d, 2Jgem = 8.0 Hz, H, CCH2); 6.37 (d, 2Jgem = 8.0 Hz, H, CCH2); 7.11 (t, 3J = 8.0 Hz, H, C6H); 7.22 (t, 3J = 8.0 Hz, H, C5H); 7.545 (d, 3J = 8.0 Hz, H, C4H); 8.08 (s, H, NCH); 8.46 (br, s, H, C7H). 13C NMR (CDCl3, 100 MHz): 28.4 (OC(CH3)3); 31.0 (OCH3); 52.5 (CH2CO); 84.1 (OC(CH3)3); 115.6 - 149.4 (CPh); 167.0 (CH2CO). HRMS (ESI): calcd. for C17H19NO4[M]+: 301.3420, found: C17H20NO4 [M + H+]: 302.1398.
Methyl 2-(1-Methyl-1H-Indol-3-yl)Acrylate 4c.
According to General Procedure 3, 0.52 g (2.56 mmol) of 2c, 0.512 g (12.79 mmol) of NaH 60% and 0.692 g (23.04 mmol) of paraformaldehyde were added. Green oil, yield 62%. 1H NMR (CDCl3, 400 MHz), δ (ppm): 3.78 (s, 3H, NCH3); 3.85 (s, 3H, OCH3); 6.11 (d, 2Jgem = 1.2 Hz, H, CCH2); 6.34 (d, 2Jgem = 1.2 Hz, H, CCH2); 7.44 (m, 3H, C4H-C5H-C6H); 7.49 (s, 1H, NCH); 7.76 (d, 3J = 7.0 Hz, H, C6H-C7H). (CDCl3, 100 MHz), δ (ppm): 33.1 (OCH3); 52.3 (CH2CO); 109.7 (C-4); 110.5 (C-3) 120.1 (C-7); 120.3 (C-6); 122.2 (C-5); 122.5 (C-CH2); 126.6 (C-CH2); 130.1 (C-2); 133.9 (Cipso-3a); 137.2 (Cipso-7a); 167.9 (CH2CO).
Dimethyl 2,4-Diphenylpentanedioate 5.
Isolated as a byproduct from 2a reaction conditions such as is shown in Table 1, Entry 1. Colorless oil, yield 55%. Erythro and Threo mixture. 1H NMR (CDCl3, 400 MHz) δ (ppm): 2.57 (m, 2H, CH2CH), 3.41 (m, 1H, CHCH2), 3.60 (s,s, 6H, CH3O), 7.27 (m, 10H, Ph). 13C NMR (CDCl3, 100 MHz) δ (ppm): 36.7 (CH2CH), 49.1 (CHCH2), 52.3 (CH3O), 127.8 (CPh), 128.0 (CPh), 128.9 (CPh), 138.2 (Cipso-Ph), 173.8 (COOCH3). HRMS: calcd. for C19H20O4[M]+: 312.1362, found: C19H20O4: 312.3470.
Methyl 3-Methoxy-2-Phenylpropanoate 6a.
Isolated as byproduct from 2a reaction conditions such as is shown in Table 1, Entry 7. Colourless oil, yield 29%. 1H NMR (CDCl3, 400 MHz) δ (ppm): 3.36 (s, 3H, CH3OCH2), 3.57 (dd, J = 8.4 Hz, J = 4.4 Hz, 1H, CHPh), 3.69 (s, 3H, CH3OCO), 3.91 (dd, J = 9.6 Hz, J = 4.4 Hz, 1H, CH2CH), 3.99 (dd, J = 9.4 Hz, J = 8.4 Hz, 1H, CH2CH), 7.35 (m, 5H, Ph). 13C NMR (CDCl3, 100 MHz) δ 52.0 (CH3OCH2), 52.3 (CHPh), 59.2 (CH3OCO), 74.4 (CH2CH), 127.8 (CPh), 128.1 (CPh), 128.8 (CPh), 135.7 (Cipso-Ph), 172.9 (COOCH3). HRMS: calcd. for C11H14O3[M]+: 194.0943, found: C11H14O3: 194.0990.
Dimethyl 2-(Methoxymethyl)-2,4-Diphenylpentanedioate 7.
Isolated as byproduct from 2a reaction conditions such as is shown in Table 1, Entry 6. Colourless oil, yield 11%. Diastereomers and enantiomers mixture. 1H NMR (CDCl3, 400 MHz) δ (ppm): 2.80 (m, 2H, CH2CH), 3.20 (s, 3H, CH3OC), 3.35 (m, 1H, CHPh), 3.45 (s, 3H, CH3OCO), 3.83 (s, 3H, CH3OCO), 3.90 (m, 2H, CH2C), 7.25 (m, 10H, 2Ph). 13C NMR (CDCl3, 100 MHz) δ (ppm): 36.9 (CH2CH), 50.0 (CHPh), 52.8 (CH3OCOCH), 53.2 (CH3OCOC), 75.7 (CH2C), 128.1 (Cipso-Ph), 136.6 (CPh), 141.0 (Cipso-Ph), 174.3 (CHC(O)OCH3), 177.4 (CC(O)OCH3). HRMS: calcd. for C21H24O5[M]+: 356.1624, found: C21H24O5 [M + Na]+: 379.1517. X-Ray crystallographic structure in Figure 1 [27] .
Methyl 2-(1-((Pivaloyloxy)methyl)-1H-Indol-3-yl)Acrylate 8d.
According to General Procedure 3, 0.52 g (1.9 mmol) of 2d, 0.38 g (9.5 mmol) of NaH 60% and 0.514 g (17.12 mmol) of paraformaldehyde were added. Green-yellow oil, yield 37%. 1H NMR (CDCl3, 400 MHz), δ (ppm): 1.14 (s, 9H, (CH3)3CO); 3.84 (s, 3H, OCH3); 6.07 (s, 2H, NCH2O); 6.13 (d, 2Jgem = 1.2 Hz, H, CCH2); 6.41 (d, 2Jgem = 1.2 Hz, H, CCH2); 7.21 (td, 3J1 = 1.2 Hz, 3J2 = 7.2 Hz, H, C6H); 7.28 (td, 3J1 = 1.2 Hz, 3J2 = 7.2 Hz, H, C5H); 7.49 (d, 3J = 8.4 Hz, H, C4H); 7.64 (s, H, NCH); 7.72 (d, 3J = 8.0 Hz, H, C7H). 13C NMR (CDCl3 100 MHz), δ (ppm): 26.9 (OC(CH3)3); 38.9 (OC(CH3)3); 52.1 (OCH3); 68.7 (NCH2O); 109.9 (C-4); 112.9 (C-3) 120.1 (C-7); 121.2 (C-6); 122.8 (C-5); 123.9 (C-CH2); 127.1 (C-CH2); 129.3 (C-2); 133.4 (Cipso-3a); 136.4 (Cipso-7a); 167.4 ((CH3)3CO); 178.0 (CH2CCO). HRMS (FAB+): calcd. for C18H21NO4[M]+: 315.3690, found: C17H20NO4 [M + H+]: 316.1537.
Acknowledgements
We are grateful to CONACYT for financial support (Project No. CB2015/256653). J.R. V.-C. gratefully acknowledges CONACYT for a scholarship.