Synthesis of New Fluorinated Amino-Heterocyclic Compounds Bearing 6-Aryl-5-Oxo-1,2,4-Triazin-3-Yl Moiety as Antimicrobial Agents ()
1. Introduction
Fluorine substituted 6-(2’-aminophenyl)-3-thioxo-1,2,4-triazin-5-one derivatives exhibit a wide spectrum of the medicinal, pharmacological and biological fields such as anti-HIV [1] , anti-cancer [2] , and antimicrobial [3] [4] activity. On the other hand, fluorinated 6-aryl-3,5-diamino-1,2,4-triazines is used as lamotrigine drugs especially as ati-inflammatory agents [5] [6] . In addition, introduction fluorine atoms to functionally 1,2,4-triazines often improve and enhance that physical, chemical and biological properties [7] [8] [9] [10] (Figure 1). Based upon these observations and in view of our previous work [6] the objective of this work is to
![]()
Figure 1. Some highly bioactive fluorine substituted 1,2,4-triazinones.
study the chemical reactivity of polyfunctional 1,2,4-triazinone used for synthetic of lamotrigine analogues drugs in view of their antimicrobial activity.
2. Chemistry
In the recent years, numerous small molecule containing a polyfunctional 1,2,4-triazine scaffold have been shown to exhibit an important properties as pharmacological, medicinal and biological effects [11] [12] [13] [14] . In search for new fluorine compounds exhibit a highly biocidal effects, the present work tends to synthesis some new fluorine substituted 3-amino-1,2,4-triazines as Lamotrigine drug analogues. Therefore, aroylation of 6-(2’-aminophenyl-3-thioxo-1,2,4-triazin-5-(2H, 4H) one (1) by warm with 4-nitrobenzoyl chloride and/or 4-methoxy benzoyl chloride in DMF afforded 6-[2’-(4’’-substituted aroyl)aminophenyl]-3-thioxo-1,2,4-triazin-5(2H, 4H) ones (2a, b) (Scheme 1).
Fluoroamination of compounds 2a, b via reflux with 4-fluoroaniline in EtOH via loss of H2S, yielded 3-(4’-fluorophenyl)amino-6-aryl-1,2,4-triazin-5(4H)ones (3a, b).
On the other hand, reflux of compounds 3 with ammonia in EtOH, furnished 5-amino-3-(4’fluorophenylamino)-6-aryl-1,2,4-triazines (4a, b) respectively as fluorinated Lamotriagine analogues drugs (Scheme 1). Reactivity of a free amino group at position 5 of 1,2,4-triazines 4 deduced from addition of phenyl isothiocyanate under reflux with DMF to produce N,N-disubstituted thioureas 5 the ring closure reaction with malonic acid in boil with glacial AcOH, afforded N’,N3-disubstituted-thiobarbituric acid 6. Finally, heterocyclization of compounds 3a, b via boiling with DMF via dehydration furnished 3-(4’-fluorophenyl)amino-5-(aroyl-1,2,4-triazino[5,6-b]indoles (7a, b) (Scheme 1).
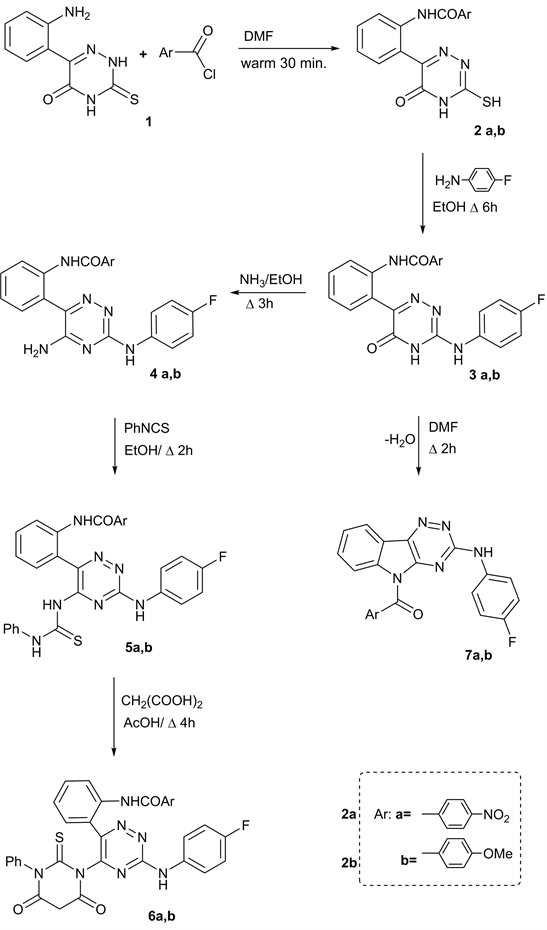
Scheme 1. Synthesis some new fluorine substituted 3-amino-1,2,4-triazines as Lamotrigine drug analogues.
3. Results and Discussion
The structures of the new produced fluorinated systems 3 - 7 have been deduced from both the correct elemental analysis and their spectral spectrum. IR spectra of both 2a & 2b recovered the disappearance of NH2 group, with an additional new carbonyl of benzamide group at 1620 cm−1, in addition the presence of the characteristic bands at ν 1530 & 1350 for asymmetric and symmetric NO2 group of 2a and ν at 1050 cm−1 for the ether group -O-Me of 2b, which deduce that structure.
On the other hand, IR absorption spectra of 4a & 4b showed the presence of ν at 3350, 3150 & 1610 cm−1, for NH2, NH and CONH functional groups, in addition of ν at 1240 - 1230 cm−1 for C-F groups, with lacks of SH functional group.
IR spectra of compounds 5a & 5b showed the presence of two ν at 3200 - 3150, 1610, 1250 cm−1 for two NH, CONH and C-F functional groups.
Also, IR spectra of 6a, 6b recorded the ν at 3150 - 3090 cm−1 for NH and 1610 - 1600 for CONH, with ν at 1240 cm−1 for C-F, in addition ν at 1520, 1350 cm−1 for asymmetric NO2 for 6a with ν at 1080 cm−1 for the ethereal group -C-O-Me. Compounds 6 showed ν at 1340 cm−1 for acyclic NCSN with ν at 2900, 2880, 1480, 1440 cm−1 for cyclic CH2 group.
IR absorption spectra of compounds 7 showed only ν at 3100, 1700, 1250 cm−1 for NH, C=O & C-F functional groups which confirm that structures.
1HNMR spectra of compounds 3 - 7 showed the resonated signals at 12 - 11 ppm for NH protons with the d d signals at 7.2 & 7.1 ppm for CH adjacent of fluorine atoms, with other aromatic protons at 7 - 6.6 ppm. Only the compounds 2a-6b showed resonated signals at δ 3.5 ppm for OMe proton present. In addition, spectra of 7a, 7b exhibited only δ at 11.0 ppm for NH proton.
13Cnmr spectra of the new compounds showed mainly (3, 4, 6 & 7) the resonated signals at δ 160 - 155 ppm for C=O, and δ at 145 ppm for C-F, in addition δ at 140 ppm for C=N, with a signals at δ 130 - 120 ppm for aromatic carbons.
Compounds 5b, 6b exhibited δ at 24 ppm for CH3O carbons. Mass fragmentation patterns of compound 4 showed that a molecular ion peak with a base peak at m/z 95 attribute to 4-fluorophenyl fragment (Figure 2).
Only the 13Cnmr of 6 recorded δ at 180, 160, 150 ppm attribute to C=S, C=O of thiobarbituric acid with δ at 40 ppm for OMe carbon.
4. Experimental
Melting points determined with an electrochermal Bibly Stuart Scientific melting point sample (UK). A Perkin Elmer Model PXI-FT system 55529 was used for recording IR spectra of the prepared compounds. A Brurker advance DPX 400 MHz model uses TMS as internal standard was used for recording the 1H and 13C NMR spectra of the compounds on deuterated DMSO-d6. A GC-MS-GP 1000 Ex model is used for recording the mass spectra of the compounds. Electronic spectra recorded in ethanol on Shimadzu uv and visible 310 IPC Spectrophotometer. Elemental analysis was performed in micro analytical center of Cairo University, Cairo, Egypt. Compound 1 obtained by the reported method [1] . Biological activity carried out in Biology center, Faculty of Science, AinShams University.
![]()
Figure 2. Mass fragmentation patterns of compound 4.
6-(2’-Aroylamino)phenyl-3-thioxo-1,2,4-triazin-5-(2H,4H)ones (2a & 2b)
A mixture of 1 [1] (0.01 mol) and 4-nitrobenzoyl chloride or 4-methyl benzoyl chloride (0.01 mol) in DMF (20 mL) refluxed for 30 min., cooled and poured onto ice. The obtained solid filtered off and crystallized from EtOH to give 2a or 2b respectively.
2a: 68%, m.p. 248˚C - 250˚C. IR (Cm−1) 2a, 3200 (NH), 1680, 1620 (CO, CONH), 1530 & 1350 (asymmetric & symmetric NO2), 1200 (C=S), 880 (substituted phenyl). 1HNMR (DMSO-d6) (δ) ppm.: 11.8 (s, 1H, NH), 8.5 (s, 1H, NHCO), 7.7 - 7.2 (m, 4H, aromatic protons), 5.5 (s, 1H, SH). 13Cnmr (DMSO-d6) (δ) ppm: 179 (C=S), 165 (C=O), 155 (CONH), 140 (C=N), 130 - 120 (aromatic carbons). Analytical data Calcd: C, 52.03; H, 2.98; N, 18.97; S, 8.67% for C16H11N5O4S (369). Found: C, 51.88; H, 2.80; N, 18.77; S, 8.55%.
2b: 88%, m.p. 232˚C - 235˚C. IR (ν Cm−1) 3180 (NH of 1,2,4-triazin), 2900 - 2880 (str. CH3), 1670, 1610 (C=O, CONH), 1580 (C=N), 1480, 1440 (bending CH3), 1180 (C=S), 820 (aromatic CH). 1 HNMR (DMSO-d6) (δ) ppm.: 11.8, 10.8, 8.9 (each s, 3 NH), 7.6 - 7.2 (m, 4H, aromatic protons), 1.25 (s, 3H, CH3). 13 Cnmr (DMSO-d6) (δ) ppm: 180 (C=S), 166 (C=O), 158 (CONH), 142 (C=N), 132 - 122 (aromatic CH), 44 (CH3-O). Analytical data Calcd: C, 57.79; H, 3.68; N, 15.86; S, 9.06% for C17H13N4OS (353). Found: C, 57.59; H, 3.60; N, 15.66; S, 8.59%.
6-(2’-Aroylamino)phenyl-3-(4’-fluorophenyl)amino-1,2,4-triazin-5-(2H)ones(3a & 3b)
A mixture of 2a or 2b (0.01 mol) with 4-fluoroaniline (0.01 mol) in EtOH (50 mL) in reflux for 6 h, cooled. The produced solid filtered off and crystallized from EtOH to give 3a & 3b respectively.
3a: 66%, m.p 350˚C. IR (ν Cm−1: 3200 - 3100 (NH, NH), 1680, 1600 (C=O, CONH), 1530, 1350 (asymmetric & symmetric NO2), 1250 (C-F), 900, 820 (substituted phenyl), 750 (C-F). 1HNMR (DMSO-d6) (δ) ppm.: 11.6, (s, 1H, NH), 10.5 (s, 1H, NHCO), 7.8 - 7.6, 7.4 - 7.25 (each m, 8H, aromatic protons), 7.1 - 6.9, 6.6 - 6.5 (dd, CH, adjacent to C-F). 13Cnmr (DMSO-d6) (δ) ppm: 160, 152 (C=O, CONH), 145 (C-F), 140 (C=N), 130 - 122 (aromatic carbons). Analytical data Calcd: C, 59.19; H, 3.36; N, 18.83; F, 4.26% for C22H15N6O4F (446). Found: C, 58.88; H, 3.15; N, 18.65; F, 4.08%.
3b: yield 82%, m.p 250˚C - 252˚C. IR (ν Cm−1: 3150 (NH), 3050 (aromatic CH), 2950, 2880 (aliphatic CH), 1680, 1620 (C=O, CONH), 1580 (C=N), 1480 (deformation CH3), 1240 (C-F), 1080 (C-O-C), 880, 820 (substituted phenyl), 720 (C-F). 1HNMR (DMSO-d6) (δ) ppm.: 11.8, 10.8 (each s, 3H, 3NH), 7.8 - 7.2 (m, 8H, aromatic protons), 7.0 - 6.8 (d,d, CH, adjacent to C-F), 1.2 (s, 3H, CH3). 13Cnmr (DMSO-d6) (δ) ppm: 160, 150 (C=O), 145 (C-F), 140 (C=N), 130 - 122 (aromatic carbons), 44 (CH3). Analytical data Calcd: C, 64.03; H, 4.17; N, 16.24; F, 4.40% for C23H18N5O3F (431). Found: C, 63.85; H, 4.01; N, 16.12; F 4.25%.
5-Amino-3-(4’-fluorophenyl)amino-6-(2’-aroylamino)-phenyl-1,2,4-triazins (4a & 4b)
A mixture of 3a or 3b (5.0 gm and liquid ammonia 20.0 mL) in EtOH (20 mL) in reflux for 3 h, cooled then drops of acetic acid were added. The resultant solid filtered off and crystallized from MeOH to give 4a & 4b respectively.
4a: 55%, m.p > 300˚C. IR (ν Cm−1: 3400 - 3100 (b, NH, NH2), 3050 (aromatic CH), 1640 (deformation NH2), 1620 (CONH), 1580 (C=N), 1520, 1350 (asymmetric & symmetric NO2), 1240 (C-F), 880, 820 (substituted phenyl), 780 (C-F). 1HNMR (DMSO-d6) (δ) ppm.: 11.6, 10.80 (each s, 2H, 2NH), 7.9 - 7.6 (m, 4H, aromatic protons), 7.3, 7.1 (d, d, 2H, CH, adjacent to C-F), 3.66 (s, 2H, NH2). 13 Cnmr (DMSO-d6) (δ) ppm: 155 (CONH), 145 (C-F), 142 (C=N), 140, 138 (C-N of 1,2,4-triazine), 130 - 120 (aromatic carbons). M/S (Int.%): 445 (447, M + 2, 10.15%); 183 (36.1); 155 (81.1); 136 (8.5), 116 (11.1) & 95 (100%). Analytical data Calcd: C, 59.32; H, 3.59; N, 22.02; F, 4.26% for C22H16N7O3F (445). Found: C, 59.11; H, 3.25; N, 21.88; F, 4.15%.
4b: yield 68%, m.p 260˚C - 262˚C. IR (ν Cm−1): 3300 - 3100 (b, NH, NH2), 3050 (aromatic CH), 1640, 1610 (CONH), 1580, 1560 (C=N), 1530, 1320 (asymmetric &symmetric NO2), 910, 840 (substituted phenyl), 750 (C-F).). 1HNMR (DMSO-d6) (δ) ppm.: 12.0, 10.8 (each s, 2H, 2NH), 7.7 - 7.2 (m, 4H, aromatic protons), 7.0 - 6.7 (dd, CH, adjacent to C-F), 3.4 (s, 2H, NH2). 13Cnmr (DMSO-d6) (δ) ppm: 155 (CONH), 145 (C-F), 145 (C-F), 142 (C=N), 138, 136 (C-N) 130 - 120 (aromatic carbons). Analytical data Calcd: C, 64.33; H, 4.19; N, 19.58% for C23H18N6O2F (429). Found: C, 64.01; H, 4.00; N, 19.39; F 4.11%.
N-(Phenyl)-N2-[3-(4’-fluorophenyl)amino-6-(aroylamino)-phenyl-1,2,4-triazin-3’-yl]thiourea (5a & 5b)
A mixture of 4a or 4b (0.01 mol) and phenylisothiocyanate (0.01 mol) in DMF (20 mL) in reflux for 2 h, cooled. The yielded solid filtered off and crystallized from EtOH to give 5a & 5b respectively.
5a: 60%, m.p 313˚C - 315˚C. IR (ν Cm−1: 3200, 3150, 3100 (NH), 3050 (aromatic CH), 1610 (CONH), 1530, 1330 (asymmetric & symmetric NO2), 1350 (NCSN), 1240 (C-F), 1188 (C=S), 910, 880, 820 (substituted phenyl), 720 (C-F). Analytical data Calcd: C, 59.38; H, 4.60; N, 19.11; F, 3.24; S, 5.46% for C29H27N8O3FS (586). Found: C, 5.11; H, 4.35; N, 19.01; F, 3.05; S, 5.30%.
5b: yield 70%, m.p 325˚C - 327˚C. IR (ν Cm−1: 3300, 3210, 3180 (NH), 3060 (aromatic CH), 2900 (aliphatic CH), 1620 (CONH), 1570 (C=N), 1350 (NCSN), 1190 (C=S), 1050 (C-O-C), 920, 870, 820 (substituted phenyl), 720 (C-F). Analytical data Calcd: C, 63.15; H, 5.08; N, 17.19, F, 3.33; S, 5.61% for C30H29N7O2FS (570). Found: C, 62.85; H, 4.79; N, 17.01; F 3.10; S, 6.36%.
N’-(Phenyl)-N3-[3-(4’-fluorophenyl)amino-6-(2-aroylamino)-phenyl-1,2,4-triazin-5’-yl]thiobarbituric acids (6a & 6b)
A mixture of 5a or 5b (0.01 mol) and malonic acid (0.01 mol) in glacial acetic acid (20 mL) in reflux for 4 h, cooled. The obtained solid filtered off and crystallized from EtOH to give 6a & 6b respectively.
6a: 55%, m.p 338˚C - 340˚C. IR (ν Cm−1: 3300, 3150 (2NH), 3060 (aromatic CH), 2950, 2880 (aliphatic CH), 1660, 1650, 1610 (3C=0), 1600 & 1580 (C=N), 1480, 1440 (deformation CH2), 1530, 1340 (asymmetric & symmetric NO2), 1240 (C-F), 1188 (C=S), 950, 910, 880, 820 (substituted phenyl), 750 (C-F). Analytical data Calcd: C, 59.16; H, 3.38; N, 17.25; S, 4.93; F, 2.92% for C32H22N8SFO5 (649). Found: C, 58.89; H, 3.15; N, 17.11; S, 4.80; F, 2.88%.
6b: 78%, m.p 325˚C - 327˚C. IR (ν Cm−1): 3280, 3210, 3150 (NH), 3080 (aromatic CH), 2960, 2880 (aliphatic CH), 1670, 1660, 1600 (3C=O), 1580 (C=N), 1330 (cyclic NCSN), 1250 (C-F), 1188 (C=S), 1100 (C-O-C), 920, 880, 820 (substituted phenyl), 720 (C-F). 1HNMR (DMSO-d6) (δ) ppm.: 12.0, 10.0, 8.5 (each s, 3NH), 7.6 - 7.4, 7.1 - 6.8, 6.6 - 6.35 (each m, 17H, aromatic protons), 4.8 - 4.66 (s, 2H, CH2), 3.5 - 3.25(s, 3H, CH3). 13Cnmr (DMSO-d6) (δ) ppm: 188 (C=S), 130 - 120 (aromatic carbons), 44 (CH3). Analytical data Calcd: C, 64.8; H, 3.88; N, 15.88; S, 5.18; F, 3.07% For C33H24N7SFO3 (617). Found C, 63.95; H, 3.59; N, 15.60; S 5.10; F, 2.85% .
3-(4’-fluorophenyl)amino-5-aroyl-1,2,4-triazino[5,6-b]indoles (7a & 7b)
Compound 3a or 3b (0.5 gm) with DMF (20 mL) reflux for 2 h, cooled then poured onto ice. The produced solid filtered off and crystallized from MeOH to give 7a & 7b respectively.
7a: 60%, m.p 328˚C - 330˚C. IR (ν Cm−1: 3100 (NH), 3050 (aromatic CH), 1700, 1580 (C=N), 1530, 1340 (asymmetric & symmetric NO2), 1240 (C-F), 890, 860, 810 (substituted phenyl). Analytical data Calcd: C, 63.04; H, 4.52; N, 20.28; F, 3.44% for C22H10N6FO3 (426). Found: C, 62.89; H, 4.18; N, 20.15; F, 3.18%. M/S (Int.%): 553 (M + 1, 5.5), 163 (1.15), 129 (88), 112 (13), 95 (100), 88 (5.11).
7b: 72%, m.p 268˚C - 270˚C. IR (ν Cm−1: 3180 (NH), 3060 (aromatic CH), 2960, 2888 (aliphatic CH), 1690 (C=O), 1580 (C=N), 1250 (C-F), 1090 (C-O-C), 890, 840, 810 (substituted phenyl), 750 (C-F). Analytical data Calcd: C, 67.16; H, 5.03; N, 18.2; F, 3.54% For C23H14N5FO2 (411). Found C, 66.89; H, 4.90; N, 18.15; F, 3.25% .
5. The Antimicrobial Evaluation
The new fluorinated 1,2,4-triazinones 3-7 were evaluated as antimicrobial agents by the use of agar well diffusion method [14] against Escherichia Coli as bacteria and against Penicillium Chrysogemuum as fungi. The Penicillin (25 μg/ mL) and mystatin (25 μg/ mL) used as antibiotic reference. DMSO (1%) also used as a control. The zones of inhibition measured in mm. The results reported in Table 1.
From the results obtained we can obtained that:
1) All the tested compounds showed the microbial activity which contain -OMe group (2-7b derivatives), showed higher activity than that contains the NO2 group (a derivatives).
2) The reduced growth activity against E. coli and or P. chrysogenum exhibit by the synthesized fluorinated 1,2,4-triazins as:
4b > 3b > 4a > 3a; 5b > 5a > 7b > 7a and 6b > 6a.
![]()
Table 1. The antimicrobial activity of the New Fluorinated Systems.
Rd: Reduced Growth; NA: Not Applicable.
3) It is clear that, a higher fluorine percentage present of the compounds, led to a more reduced growth of these microorganisms.
4) The most active fluorinated compounds 4a & 4b, which have a similar activity of lamotrigine drug.
6. Conclusion
Inspired by Lamotrigine drug constitutional and in a search of new high biocidal agents, fluorine substituted 1,2,4-triazines scaffolds have been synthesized via a simple methods with good yield. These compounds have been evaluated as antimicrobial agents as isomeric structural to lamotrigine, which showed a high activity, especially in the presence of -OMe groups, in addition to fluorine atoms. Further biological evaluation could screen the reactivity of few products containing thioxodihydropyrimidine-4,6(1H,5H)-dione(6) a moiety similar to thiobarbituric acid.