Towards Biorefinery Production of Microalgal Biofuels and Bioproducts: Production of Acetic Acid from the Fermentation of Chlorella sp. and Tetraselmis suecica Hydrolysates ()
1. Introduction
Microalgae have significant advantages over traditional food crops as biofuel sources because of their high area yields, non-requirement for agricultural resources (arable land and clean water) and their potential as a CO2 bio-sequestra- tion platform to capture flue gas released from power plants [1] . In addition, microalgae also comprise other components (such as pigments, ω3 fatty acids, protein) that can potentially be transformed into high-value nutraceutical and biochemical products in a biorefinery integrated system [1] .
Different types of biofuels (such as biodiesel and alcohol) can be produced from microalgal biomass depending of which component of the biomass is utilized [2] . Bioethanol or biobutanol is produced through anaerobic fermentation of microalgal carbohydrates, while biodiesel is produced through transesterification of extracted microalgal lipids [3] . Carbohydrate in microalgal cells is generally found as starch in the chloroplasts and cellulose or polysaccharides in the cell wall. Cultivation conditions, such as light intensity, temperature and salt concentration, have previously been shown to have a significant effect on microalgal carbohydrate content and composition [4] [5] .
According to recent life cycle analyses, the commercialisation of microalgal biotechnology requires the development an integrated biorefinery production system that is able to utilise all of the components of the biomass and convert them to biofuels, high-value products and chemicals [2] [6] . The biorefinery approach reduces the average cost of producing a single product and improves the economic prospect of microalgal bioindustry. A microalgal production strategy that is solely focused on a single biofuel conversion is generally considered to be lacking in both economic and energetic feasibility [2] [6] . For this reason, research on microalgal biofuels has to focus on finding strategies that will facilitate the co-production of biofuels, high-value nutraceuticals (such as chlorophyll antioxidants) and industrial products (such as organic acids) from the biomass.
Acetone, butanol, ethanol (ABE) fermentation with the gram-positive bacterium C. saccharoperbutylacetonicum has previously been applied to microalgal biomass to produce butanol [7] . The bacterium has two distinct phases of anaerobic metabolism. During logarithmic growth, it performs acidogenesis fermentation which produces butyric acid and acetic acid as major products. At early stationary phase, a major metabolic shift driven by imminent threat of cell death due to low pH takes place. The bacterial cells start performing solventogenesis fermentation, where excreted acids from previous acidogenesis phase are re-as- similated to increase solution pH and converted to more neutral products, such as butanol and ethanol. The switch between the acidogenesis and solventogenesis pathways is reliant on growth phase, which in turn is dependent on fermentation conditions and initial sugar concentration in the solution [8] [9] . In this study, we are interested to explore the use of ABE fermentation such that it can be tuned to enable the production of industrially important biochemical, e.g. acetic acid and butyric acid, from microalgal sugar. Such production will add significant commercial values to existing microalgal biodiesel production frame- work and can potentially be used to design a novel and cost-effective microalgal biorefinery system.
This study investigated the use of sugar hydrolysate obtained from enzymatic saccharification of microalgal biomass (Chlorella sp. and Tetraselmis suecica) as a fermentation feedstock for the production of industrial chemicals. In its first phase, the study evaluated the optimal cultivation conditions needed to achieve maximum biomass concentration and carbohydrate content for each species. In the second phase of the study, microalgal biomass grown under optimal conditions from the first phase of the study was subjected to enzymatic saccharification in order to break down complex carbohydrates in the cells and release them in the form of reducing sugars. The hydrolysate was collected and subjected to anaerobic fermentation with bacterium C. saccharoperbutylacetonicum. We tuned the fermentation conditions to prevent solventogenesis shifting, thus ensuring that the fermentation resulted in the production of organic acids. The extent of ABE fermentation in converting reducing sugar in the microalgal hydrolysate to acetic acid and other organic products was analyzed. To the best of our knowledge, this is the first study that examines the production of acetic acid and butyric acid from the fermentation of microalgal hydrolysate. The formation kinetics of the various fermentation products were fitted to a modified sigmoidal function (Gompertz equation).
2. Materials and Methods
2.1. Microalgae, Medium Preparation and Chemical Reagents
Two different microalgal species, Chlorella sp. and Tetraselmis suecica, were used in this study. Chlorella is a freshwater species and spherical in shape, while T. suecica is a marine strain and elliptical in shape. Both Chlorella sp. and T. suecica are industrially relevant microalgals rains for their relatively high lipid contents and growth rates. Modified algae growth medium with 0.49 g/L magnesium sulphate (MgSO4・7H2O), 1.7 g/L sodium nitrate (NaNO3), 0.14 g/L di-potassium phosphate (K2HPO4), 0.03 g/L calcium chloride (CaCl2・2H2O) was used for the cultivation of both species. The medium was sterilised using 0.22 µm Millipore filter.
Chemical reagents for MLA medium formulation, standards for GC analysis (acetone, butanol, ethanol, acetic acid and butyric acid) and other reagents used throughout the study, such as sodium hydroxide, potassium hydroxide, hydrochloric acid and 3, 5 dinitrosalysilic (DNS) acid, were obtained from commercial suppliers. The enzyme cocktail used for cell wall saccharification was purchased from Sigma-Aldrich (as described in section 2.3.1).
2.2. Optimisation of Cultivation Parameters
The effect of three cultivation conditions on microalgal growth and carbohydrate accumulation were investigated: light intensity, temperature and NaCl concentration. For each species, microalgal culture was inoculated at 10% v/v. Biomass concentration was monitored every 24 h for 10 days. A sample of the biomass was also harvested every 24 h for carbohydrate analysis. Each experiment was carried out in triplicate.
2.2.1. Effect of Light Intensity
To study the effect of light intensity, each species was grown under the following light intensities: 0, 60, 120 and 300 μmol/(m2s). Light intensity was monitored using a quantum meter (Spectrum Technologies Inc, USA). MLA medium with an initial pH of 7 and 30% (w/v) dissolved NaCl was used. Cultivation was carried out at 20˚C for 10 days.
2.2.2. Effect of Temperature
To study the effect of temperature, each species was grown at four different temperatures: 20˚C, 25˚C, 30˚C and 40˚C. MLA medium with an initial pH of 7 was used and cultivation was carried out at the optimal light intensity as determined from section 2.3.1 for 10 days.
2.2.3. Effect of Salinity
To study the effect of salinity, each species was grown at five salinity levels; 0, 10, 20, 30 and 40 g/L of NaCl. MLA medium with an initial pH of 7 was used. Cultivation was carried out at the optimized light intensity and temperature obtained from the previous sections.
2.2.4. Growth and Carbohydrate Content Determination
Biomass concentration was determined by measuring optical density (OD) of the culture at 680 nm. The following equations describe the relationship between biomass concentration and OD680:
XChlorella sp. =0.549(OD680) − 0.0046 (1)
XT. suecica = 0.524(OD680 ) − 0.0129 (2)
where X is the biomass concentration (g/L).
Harvested culture was centrifuged at 4500 rpm for 15 min. The pellet was rinsed twice with distilled water and dried at 70˚C for 24 h. The total carbohydrate content of the dried microalgal biomass (Ycarb) was determined using phenol?sulfuric acid method [10] .
2.3. Fermentation of Microalgal Hydrolysates
2.3.1. Dilute Alkaline Pre-Treatment, Enzymatic Saccharification and ABE Fermentation
Alkaline pre-treatment using a total of 1.0 g of dried microalgal biomass was performed as described by Kassim & Bhattacharya [11] . Pretreatment of Chlorella sp. biomass was carried out with 2% (w/v) sodium hydroxide (NaOH) at 120˚C for 30 min. Meanwhile, pretreatment for T. suecica biomass was performed using 2% (w/v) of potassium hydroxide (KOH) at 120˚C for 120 min. The mixture was then cooled to room temperature before being centrifuged at 3000 rpm for 10 min. Microalgal pellet was neutralized by repeated washing with hot water until pH value of 7 was obtained. The pellet was dried overnight for enzymatic saccharification.
For enzymatic saccharification, alkaline-pretreated microalgal biomass was soaked in 100 ml of 10 mM acetate buffer (pH 5.5) and mixed with complex multienzymecellulase cocktail produced from Trichoderma longibrachiatum. The enzyme cocktail consisted of xylanase, pectinase, mannanase, xyloglucanase, laminarase, β-glucosidase, β-xylosidase, α-l-arabinofuranosidase and amylase (Sigma?Aldrich C9748). The activity of the enzyme was 20 FPU/g biomass. The saccharification was carried out at 50˚C and 150 rpm in an orbital shaker (Thermoline Scientific) for 72 h. A total of 1.0 mL of the sample was withdrawn every 24 h and heated at 100˚C to deactivate the enzymatic activity. The sample was then centrifuged at 3500 rpm for 5 min and the supernatant was used for sugar analysis. Enzymatic saccharification was performed in duplicate.
90 mL of the hydrolysate obtained from enzymatic saccharification process was used as the medium for ABE fermentation. The pH value of the hydrolysate was adjusted to 6.0 using 1 M hydrochloric acid (HCl) or 1 M sodium hydroxide (NaOH) followed by sterilization with autoclaving at 120˚C for 10 minutes. After sterilization, anaerobic condition was induced by passing nitrogen gas through the medium for about 5 min. The hydrolysate was then inoculated with 10% active Clostridium saccharoperbutylacetonicum N1-4. The mixture was incubated at 35˚C for 96 hours in an oven. Sample was withdrawn at regular intervals throughout ABE fermentation to monitor sugar level and chemical concentration.
2.3.2. Chemical Analysis
Total reducing sugar was determined using 3, 5 dinitrosalysilic (DNS) acid method. Sample collected from either enzymatic saccharification or ABE fermentation was mixed with 1 mL of DNS reagent and then boiled for 10 min in the water bath. The reaction mixture was allowed to cool and reducing sugar concentration was estimated at 540 nm with UV spectrophotometer (Hach, DR-5000). Fermentation products (acetone, butanol, ethanol, acetic acid and butyric acid) in the hydrolysate of ABE fermentation were determined with gas chromatography (Shimadzu-1200, Japan) equipped with an HP-FFAP capillary column (Agilent, USA). The oven temperature of the GC was programmed to increase from 50˚C to 200˚C at a rate of 20˚C/min. The injector and detector temperature were set at 260˚C. Helium was used as a carrier gas and was set at a flow rate of 29 mL/min. Concentration of different fermentation products was quantified by comparison with standard calibration curves.
2.3.3. Kinetic Parameters for Anaerobic Fermentation
A modified Gompertz equation incorporating exponential lag time was employed to model the formation kinetics of individual product arising from ABE fermentation (ethanol, butanol, acetone, acetic acid and butyric acid) [12] [13] .
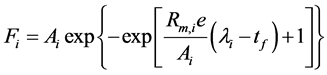 (3)
(3)
where i represents individual fermentation product, Fi is the amount of product i formed per unit volume of the fermentation mixture (g/L), Ai is the potential maximum amount of product i formed per unit volume of the fermentation mixture (g/L), Rm,i is the maximum production rate for product i (g/L/h), λi is the lag time of product i to exponential formation phase (h) and tf is fermentation time (h). Data fitting and regression work to calculate the Gompertz constants (Ai, Rm,i and λ) for each fermentation run were carried out with solver function in MS Excel 2010 (Microsoft, Inc., US). The Gompertz model describes sigmoidal kinetics with less symmetry around the inflection point.
3. Results and Discussion
3.1. Effect of Cultivation Conditions on Microalgal Growth and Carbohydrate Accumulation
During ABE fermentation, the complex carbohydrate in the microalgal biomass was used as substrate for the production of solvents and organic acids. For this reason, it is of interest to understand how carbohydrate content of the biomass varied under different cultivation conditions. In this phase of the study, cultivation conditions that led to the highest growth rate and carbohydrate accumulation were evaluated for both species.
Increasing light intensity improves photosynthetic rate and increases biomass production. Overexposure to light, however, can adversely affect growth as it may lead to irreversible damage to the chloroplasts and lamellae in photosynthetic apparatus (known as photoinhibition). The optimal light intensity is known as light saturation level [14] [15] [16] . Figure 1 shows the growth profile and carbohydrate content of Chlorella sp. and T. suecica cultivated under four different light intensities: 0, 60, 120, and 300 μmol/(m2s). Light saturation level for Chlorella occurred between 60 and 120 μmol/(m2s); When subjected to light intensity within this range, the species achieved its highest biomass concentration (X = 0.35 ± 0.02 g dried biomass/L) and carbohydrate content (Ycarb = 30.56 ± 0.39 wt% of dried biomass). Light saturation level for T. suecica was not found within the range of light intensities investigated in the study. Biomass concentration and carbohydrate content for the species continued to increase with light intensity, obtaining their maximum values (X = 0.51 ± 0.07 g/L and Ycarb value of 37.67 ± 0.62 wt% of dried biomass) at 300 μmol/(m2s).
Figure 2 shows the growth profile and carbohydrate content of both microalgal species at four different cultivation temperatures: 20˚C, 25˚C, 30˚C and 40˚C. Both species achieved their highest biomass concentrations (X for Chlorella =
![]()
Figure 1. Microalgal growth profile and carbohydrate content (wt% of dried biomass) under different light intensities: (a) Growth profile for Chlorella sp., (b) Growth profile for T. suecica, (c) Carbohydrate content of Chlorella sp. and T. suecica.
![]()
Figure 2. Microalgal growth profile and carbohydrate content (% of dried biomass) at different cultivation temperatures: (a) Growth profile for Chlorella sp., (b) Growth profile for T. suecica, (c) Carbohydrate content of Chlorella sp. and T. suecica.
0.49 ± 0.02, X for T. suecica = 0.53 ± 0.013 g/L) and carbohydrate contents (Ycarb for Chlorella = 31.86 ± 1.36, Ycarb for T. suecica = 25.14 ± 1.14 wt% of dried biomass) at 30˚C, a temperature generally known to be ideal for enzymatic activities and cellular nutrient uptake. At 40˚C, the growth of both species was significantly reduced. The growth retardation could be attributed to enzymatic denaturation and protein damage of the photosynthetic reaction centre [17] [18] [19] .
Figure 3 shows the growth profiles and carbohydrate contents of Chlorella sp. and T. suecica cultivated in different NaCl concentrations: 0, 10, 20, 30 and 40 g/L. The highest biomass concentration and carbohydrate content for Chlorella sp. were obtained when the cells were grown without NaCl (X = 0.61 ± 0.08 g/L). For Chlorella, an increase in medium salinity was found to result in reduced biomass concentration. Biomass concentration for culture grown in 40 g/L of NaCl was 0.15 g/L, less than a quarter of biomass concentration for culture grown in medium with no NaCl. Since the Chlorella sp. used in this study was a freshwater strain, it was anticipated to lack the evolutionary adaptability required to acclimate to a high-salinity environment [20] [21] .
In contrast, the highest biomass concentration for T. suecica (Xm = 0.53 ± 0.02 g/L) was obtained when the species was grown in a medium that imitated the salinity of seawater (30 g/L of NaCl). When grown in a medium with salinity lower or higher than 30 g/L, the species exhibited growth retardation. Our results confirmed that T. suecica, being a marine species, was more adapted to grow in high-salinity environment than freshwater Chlorella sp [22] [23] [24] .
In summary, for Chlorella sp., moderate level of light illumination (120 μmol/ (m2s)) was ideal for optimum growth and carbohydrate accumulation, while T. suecica required more intense light illumination (300 µmol/(m2s)) to achieve its optimal growth. For both species, low light intensity (<120 μmol/(m2s) was shown to reduce photosynthetic activities which resulted in slower growth rate. Both species experienced optimum growth and carbohydrate production at 30˚C. Low cultivation temperature resulted in slower enzymatic activities and cellular nutrient uptake, while high cultivation temperature promoted enzymatic denaturation and catabolic degradation. On the basis of results obtained from this study, a low salinity level (0 g/L NaCl) was appropriate for freshwater Chlorella sp., while marine T. suecica required salt concentration similar to that of seawater (30 g/L NaCl). Cultures grown at these optimum conditions were harvested and used for our subsequent fermentation experiments.
3.2. Enzymatic Saccharification and Hydrolysate Fermentation
The primary aim of this study is to determine the potential of microalgal sugar as a feedstock for the production of industrial chemicals. The co-production of industrial chemicals in a microalgal biorefinery system will help offset the high economic cost currently associated with microalgal biofuel production and improve the economic and commercialization prospect of microalgal bio-industry. We have chosen to work on ABE fermentation using the bacterium C. saccharo-
![]()
Figure 3. Microalgal growth profile and carbohydrate content (% of dried biomass) at different NaCl concentrations: (a) Growth profile for Chlorella sp., (b) Growth profile for T. suecica, (c) Carbohydrate content of Chlorella sp. and T. suecica.
perbutyliticum. Anaerobic fermentation using this bacterial species was known to have dual acidogenesis and solventogenesis pathways that are activated under different growth phase. The acidogenesis pathway, activated in logarithmic phase, produces organic acids (such as acetic acid and butyric acid), while the solventogenesis pathway, activated during stationary phase, produces solvents (such as acetone, ethanol and butanol) [8] [9] . The range of potential chemicals that can be produced from the ABE fermentation and the relatively mild pro- cessing conditions associated with the process makes it highly attractive to be applied as a possible value-adding auxiliary step in a microalgal biodiesel production system. To this end, we subject our microalgal biomass to a series of downstream steps: dilute alkaline pretreatment to partially hydrolysemicroalgal cell wall, enzymatic saccharification to break complex carbohydrates into fermentable reducing sugar and ABE fermentation of the released reducing sugar in the hydrolysate with the bacterium C. saccharoperbutyliticum.
Figure 4 shows the yield of reducing sugar as a function of saccharification time. At the end of the 72 h, approximately 77.23% ± 0.83% and 95.85% ± 2.12% of all carbohydrates available in Chlorella sp. and T. suecica biomass respectively had been converted to reducing sugar. A higher reducing sugar conversion was observed for T. suecica compared to Chlorella sp. This discrepancy could be attributed to the cell wall composition of the two microalgal species. According to Bohutskyi et al. [25] , the cell wall of T. suecica consists mainly of glycoprotein, a substance known to be easier to hydrolyse than chitin and cellulose, the primary constituents of the cell wall of Chlorella sp. [26] .
Figure 5 shows the fermentation yield and compositions for Chlorella sp. and T. suecica hydrolysate as a function of ABE fermentation time respectively. For both species, the decrease in the concentration of reducing sugar in the hydrolysate
![]()
Figure 4. Enzymatic saccharification yield of alkaline-pretreated microalgal biomass.
![]() (a)
(a)![]() (b)
(b)
Figure 5. Yield and compositional breakdown of microalgall biomass fermentation products as a function of fermentation time. The proportion for each product is given as wt% of total fermentation products at the given fermentation time. (a) Chlorella sp. (b) T. Suecica.
was accompanied by an approximately first-order rise in the total concentration of fermentation products. The fermentation process appeared to consume the reducing sugar from T. suecica more effectively than that from Chlorella sp. Reducing sugar in T. suecica fermentation mixture was almost completely depleted after 72 h of fermentation (reducing sugar concentration at 72 h = 8% of initial reducing sugar concentration), while that for Chlorella sp. experienced a more moderate decrease (reducing sugar concentration at 72 h = 79% of initial reducing sugar concentration). By the end of the fermentation, the hydrolysate for Chlorella sp. contained 1.56 g/L of fermentation products, while that for T. suecica consisted of 0.97 g/L of fermentation products.
Table 1 compares the fermentation yield obtained from this study to those reported in the literature for various microalgal species. Even though our fermentation yield was within range of those reported in previous studies (fermentation yield of this study = 0.08 - 0.11, fermentation yield of other studies = 0.027 - 0.35 g fermentation products/g sugar), the butanol yield obtained in the study was significantly lower (butanol yield of this study = 0.03 - 0.07, butanol yield of other studies = 0.8 - 13.2 g/L). This was expected as the concentration of initial reducing sugar in our hydrolysate (0.44 g/L for Chlorella sp. and 1.52 g/L for T. Suecica) was significantly lower compared to other studies which typically had between 7.8 and 89.1 g/L of initial sugar concentration in their fermentation
![]()
Table 1. Summary of previous studies on ABE fermentation of various microalgal and macroalgal biomass. Our study is included as comparison.
medium. At this initial sugar concentration, C. saccharoperbutyliticum was unable to enter its stationary phase and to activate its solventogenesis pathway. As a result, the acidogenesis phase persisted and the fermentation resulted in a product stream that was dominated by organic acids (up to 95 wt% of total fermentation products for Chlorella and up to 85 wt% of total fermentation products for T. suecica). Dürre [8] and Kotai et al. [9] speculated thata minimum of 15 - 20 g/L of initial sugar concentration was required in order to induce metabolic shifting from acidogenesis to solventogenesis in Clostridium anerobic metabolism. The low sugar content of our hydrolysate was the result of adding excessive buffer during our saccharification step.
As shown in Figure 5, after 72 hr, the major products arising from ABE fermentation of both Chlorella sp. and T. suecica hydrolysates were acetic acid (74.32 - 92.42 wt% of total fermentation products) and butyric acid (2.77 - 10.93 wt% of total fermentation products) with small amounts of ethanol (2.71 - 5.25 wt% of total fermentation products), butanol (1.42 - 7.43 wt% of total fermentation products) and acetone (0.68 - 2.08 wt% of total fermentation products) being co-generated. Acetic acid is an industrially important chemical with major uses in the ink, paint, coating, food, textile and medical industry.
Figure 6 shows the butanol formation kinetics for T. suecica. A modified sigmoidal equation known as Gompertz function was fitted to the kinetic data and appeared to be in agreement with the butanol formation kinetics (r2 = 0.98). In fact, we fitted the Gompertz equation to the formation kinetics of each of the fermentation product across both species and found the model to correlate reasonably well with individual formation data (0.72 < r2 < 0.99). For this reason,
![]()
Figure 6. The formation kinetics of a specific fermentation product as modeled by Gompertz equation. The data shown are for butanol formation during fermentation of T.suecica hydrolysate. The three phases of specific product formation are described by the three constants in Gompertz equation: the lag time (λi) describes the lag phase and is defined as t-intercept of the tangent to the inflection point, the maximum production rate for the specific product (Rm,i) describes the exponential phase and is defined as the gradient of the tangent in the inflection point, the maximum concentration of specific product (Ai) describes the stationary phase and is defined as be asymptotic value of the curve. R2 between experimental data and model prediction = 0.98.
we concluded the Gompertz equation to be a suitable mathematical tool for describing and predicting the formation kinetics of individual product during ABE fermentation. Table 2 reports the values of the kinetic parameters obtained from the data fitting exercise.
With the Gompertz model, the formation kinetics for any specific product is divided into three distinct phases: 1) a lag phase where hardly any product is formed, 2) an exponential phase where rapid product formation is observed and 3) a stationary phase where product formation slows down (Zwietering et al., 1990). As shown in Figure 6, the Gompertz constants provide mathematical definition for each phase. The lag time to exponential phase (λi) describes the length of the initial delay phase and is mathematically defined as the t-intercept of the tangent to the inflection point of the kinetics curve. The maximum production rate (Rm,i) describes the highest instantaneous rate of product formation during the exponential phase and is mathematically defined as the gradient of the tangent to the inflection point of the kinetics curve. Maximum product concentration (Ai) describes the final product concentration in the fermentation medium at stationary phase and is mathematically defined as the asymptotic value that the kinetics curve levels to. As expected, the primary product from our ABE fermentation, acetic acid, obtained the highest Ai value for both Chlorella sp. (1.296 g/L) and T. suecica (0.684 g/L) upon data fitting.
Figure 7 shows a proposed schematic for microalgal biorefinery that integrates the use of our enzymatic saccharification and ABE fermentation for the co- production of biodiesel, ω3 fatty acids and organic acids (in particular acetic acid). The schematic combines our current study with a previous work by Halim et al. [32] which designed a novel microalgalbiorefinery system based on the separation of hexane/cell debris mixture for biodiesel production. In this schematic, highly concentrated microalgal paste is subjected to mechanical cell rupture by
![]()
Table 2. Gompertz kinetic parameters for specific product formation during ABE fer- mentation of microalgal hydrolysate. The results of data fitting for both Chlorella sp. and T. suecica are presented.
Ai = maximum concentration of product i in the fermentation mixture; Rm,i = maximum production rate for product i; λi = lag time of product i to exponential formation phase.
![]()
Figure 7. Proposed schematics of an integrated microalgal biorefinery systemfor the co-production of biodiesel, ω3 fatty acids and acetic acid. The scheme uses the enzymatic saccharification and ABE fermentation pathway described in the study as a biomass valorization step that produces biochemicals from lipid-extracted cell debris.
high-pressure homogenization and lipid extraction by hexane before being fractionated into several layers by centrifugation. Each of the layers has a distinctive composition and can be targeted for a different application, with the neutral-li- pid rich top hexane layer used for biodiesel conversion and ω3-fatty-acid rich middle emulsion layer used for high-value nutraceutical production. The bottom layer contains lipid-depleted cell debris with high polysaccharide content, serving an ideal feedstock for anaerobic fermentation. The layer can thus be directly subjected to the enzymatic saccharification and ABE fermentation steps for the production of acetic acid and butyric acid. Under the proposed biorefinery scheme in Figure 7, the ABE fermentation pathway described in this study is used as a biomass valorization step to produce high-value biochemicals from lipid-extracted cell debris.
4. Conclusions
The study investigated the use of sugar hydrolysate obtained from enzymatic saccharification of microalgal biomass (Chlorella sp. and T. suecica) as a fermentation feedstock for the production of acetic acid, an important biochemical in the polymer, paint and food industry. ABE fermentation of microalgal hydrolysate with low sugar content (<2 g/L) using the bacterium C. saccharoperbutylacetonicum was shown to follow acidogenesis pathway and generated a product stream that was dominated by organic acids (acetic acid and butyric acid) rather than alcohols (ethanol and butanol). Acetic acid emerged to the primary product for the hydrolysate fermentation of both species (up to 92 wt% of Chlorella’s fermentation products and 80 wt% of T. suecica’s fermentation products). To our knowledge, this is the first study to focus on the production of acetic acid from microalgae without subjecting the biomass to hydrothermal liquefaction. The study achieved reasonable yields of fermentation products: 0.08 g fermentation products/g sugar for T. suecica and 0.11 g fermentation products/g sugar for Chlorella. The anaerobic fermentation was able to consume more than 90 wt% of available sugar in the T. suecica hydrolysate. The Gompertz equation, a modified logistic model, was successfully used to describe the formation kinetics of individual fermentation product. The process described in the study (enzymatic saccharification and ABE fermentation) could potentially be used to produce high-value biochemicals from cell debris in an integrated microalgal biorefinery system.
Acknowledgements
This work has been supported by Universiti Sins Malaysia (USM) under USM Academic Staff Training Scheme (ASTS), the Ministry of Higher Education, Malaysia and the Department of Chemical Engineering, Monash University, Australia.
List of Terms
Ai: maximum concentration of product i in the fermentation mixture [g/L]
Fi: concentration of product i in the fermentation mixture [g/L]
λi: lag time of product i to exponential formation phase [h]
OD680: optical density of the microalgal culture at 680 nm [?]
Rm,i: maximum production rate for product i [g/L/h]
tf: fermentation time [h]
X: biomass concentration at t days of cultivation [g/L]
Ycarb: total carbohydrate content of the microalgal biomass [g carbohydrate/g dried biomass]
Conflict of Interest
The authors declare that they have no conflict of interests.
Author’s Contribution
M.M. Kassim carried out the experiments, analysis, interpretation of the data and drafted the manuscript. M.A. Rashid was involved in carrying some components of the study. R. Halim performed data fitting and revised the manuscript. All the authors read and approved the final manuscript.