Effect of Combining Ultrasound and Mild Heat Treatment on Physicochemical, Nutritional Quality and Microbiological Properties of Pineapple Juice ()
1. Introduction
Considerable attention has been focused on vegetable and fruit juices recently due to the presence of health promoting compounds in the juices and ease of consumption especially for packaged juices. Juices play an important role in the daily nutrition and provide essential supplements. Pineapple (Ananas cosmosus) is one of the most important tropical fruits. Pineapple juice is famous for its sweet and sour taste as well as beneficial health compounds. The protective effects of this product have been associated with the presence of antioxidant compounds [1] [2] , which include ascorbic acid, bromelain, carotenoids, phenolic compounds and flavonoids among others [3] . Besides, it contains sufficient amount of minerals especially potassium and calcium. Being rich in antioxidants, pineapple juice has been proven to reduce the incidence of cardiovascular disease and some chronic and degenerative diseases associated with oxidative damage [4] . Bromelain is the major proteolytic enzyme complex that is found prominently in pineapple [5] . Hale et al. [5] also reported that proteolytic activity is required for the therapeutic effect of bromelain. Bromelain exhibits therapeutic and pharmacological effects such as anti-inflammatory tumour growth modulation and aids in digestion [6] [7] .
Spoilage in pineapple products is not only encountered by microbial contamination but also by enzymatic degradation which is generally not accepted by the consumer [8] .
Thermal pasteurization is a common process used to inactivate pathogenic bacteria and some enzymes in juice. However, due to the high temperature used in the process, the nutritional and sensory properties of the pasteurized juice might be somewhat altered [9] . Thus, there is an increased demand for new methods that will have a reduced impact on the nutritional content and the overall food quality. Some non-thermal pasteurization methods have been proposed during the last couple of decades, including high hydrostatic pressure, high pressure homogenization, pulsed electric field, radiation processing, and ultrasound [10] [11] . These emerging techniques seem to have the potential to provide “fresh-like” products, and preserve nutritional and organoleptic qualities and safe fruit juices with prolonged shelf-life.
From scientific literature, it is apparent that some individual non-thermal methods are effective to inactivate microorganisms or reduce the log colony forming units (CFU) while not adversely affecting the sensory and nutritional quality.
Sonication (ultrasound) treatment, which is an emerging technology that is considered to be inexpensive, simple, reliable and environmentally friendly, has been studied for use in several applications including fruit juice processing [12] [13] [14] . According to O’Donnell et al. [15] ultrasonic processing of fruit juices has the minimal effect on the quality of fruit juices such as orange juice [14] , guava juice [16] and strawberry juice [17] . Ultrasound alone and/or in combination with other techniques has been reported to be effective against Escherichia coli in model fluids [18] , as well as apple cider [19] and against Listeria monocytogenes in apple cider [20] . Piyasena et al. and Jiranek et al. [21] [22] have reported an extensive analysis of the potential of ultrasound for inactivation of food borne pathogens.
Despite the numerous published reports on the effect of non-thermal treatments combined with mild heat pasteurization on fruit juices, there is a lack of information on pineapple juice. There is a need for alternative combined treatments that can preserve quality properties of pineapple juice. Therefore, the aim of this study was to evaluate the effect of mild heat pasteurization, thermal pasteurization, ultrasound, and their combination on the physico-chemical, microbiological properties and nutritional quality of pineapple juice during storage.
2. Materials and Methods
2.1. Sample Preparation
Freshly harvested pineapples (Ananas comosus) were purchased from a local market at Cotonou, Benin. The obtained fruits were washed, peeled and pressed mechanically in a household juice extractor. The extracted juice was further filtered using a vacuum filtration unit through Whatman filter paper No.1 and then stored in an aseptic manner and further subjected to different thermal and non-thermal treatments.
Pineapple juice obtained was divided into five groups, and each group was samples (250 ± 5 mL) at least three times using glass jars.
2.2. Experimental Design
The different groups were subjected to the following treatments.
Untreated groups are the fresh pineapple juice without any treatment was used as control (C).
Water bath having a polycarbonate basin was used for pasteurizations treatments of juices. The Biobase water bath had special features such as thermostatic control, temperature resistance till 120˚C, operating temperature range of 25˚C - 100˚C with accuracy of ±0.3˚C and electric supply of 220 V/50 Hz. For mild heat pasteurization (MP), juice (250 ± 5 mL) was transferred into a clean sterile closed glass jar and was pasteurized at 65˚C temperature for 15 min. On the other hand, for thermal pasteurization (TP), juice (250 ± 5 mL) was transferred into a clean sterile closed glass jar and subjected to heat treatment at 80˚C for 15min. The jars were kept for constant shaking at 100 rpm to obtain homogenous conditions inside the samples throughout the treatment. Samples were cooled immediately after the heat treatment and stored at room temperature (26˚C ± 2˚C) till further analysis.
Ultrasound (US) treatments were carried out using Bioblock Scientific, Vibra-cell 75,115 (with probe diameter of 10 mm) at constant power of 500 W and frequency of 20 kHz for 15 min. The temperature was maintained below 65˚C using an ice bath around the reactor. The temperature of the juice was monitored using a thermometer and it was ensured that the temperature was below 65˚C.
Juices were also treated in a combined operation using ultrasound and mild heat pasteurization (UMP). Ultrasound parameters were set at the same optimized values as mentioned earlier. After ultrasound treatment, the juice was pasteurized at 65˚C for 15 min.
The processed juice was stored at room temperature (26˚C ± 2˚C) and used at regular intervals for different analysis. Measurements and analyses of the juices were performed on the following days of storage period; 0, 15th, 30th, 45th and 60th day. Three samples were prepared for each treatment. The experiment was done in triplicates.
2.3. Physico-Chemical Analyses of Pineapple Juice
2.3.1. Determination of pH, Titratable Acidity (TA) and Total Soluble Solids (TSS)
The pH of the pineapple juice was measured using hand digital pH meter (Eutech Instruments, pH 2700, Singapore), precalibrated with buffers of pH 4.0 and 7.0.
Titratable acidity of the juice was measured by titrating with 0.1 N NaOH and the results were expressed as percentage of citric acid [23] . Total soluble solids in the juice were determined with a hand digital refractometer (Digit-032) at 20˚C. The instrument was calibrated with distilled water before the analysis. The TSS values were expressed as ˚Brix [24] .
2.3.2. Determination of Browning Degree
The browning degree (BD) of pineapple juice was analyzed using a spectrophotometric method described by Roig et al. [25] with some modification. Pineapple juice was centrifuged with a refrigerated Centrifuge (Thermo Scientific Heraeus Megafuge 16 R, Germany) at 9000× g at 4˚C for 30 min, and then passed through a 0.45 µm cellulose nitrate membrane. The BD was determined by measuring the A (absorbance at 420 nm) value using a spectrophotometer (UV-1600PC, Shanghai, China) at an ambient temperature (20˚C ± 1˚C) with a 1 cm pathlength cell.
2.3.3. Pectin Methylesterase Activity
Pectin methylesterase (PME) activity was measured using the method adapted by Aguiló-Aguayo, et al. [26] . Briefly, 10 mL of pineapple juice was added to 40 mL of 1% citrus pectin solution in 2 N NaCl and incubated at 30˚C ± 1˚C in a water bath. When the temperature of the mixture reached 30˚C, the pH of the mixture was adjusted to 7.7 (optimum pH) with 2 N NaOH using a pH meter (Eutech Instruments, pH 2700, Singapore). When a stable pH was obtained, 0.1 mL of 0.05 N NaOH was added, and the time required for the pH to return to 7.7 was recorded. PME activity was calculated using Equation (1). PME activity was defined as the amount of enzyme that liberated 1.0 µmol equivalent of acid per minute under the assay conditions.
 (1)
(1)
where [NaOH] is NaOH concentration (0.05 N); 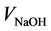 is the volume of NaOH used (0.10 mL);
is the volume of NaOH used (0.10 mL); 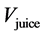 is the volume of juice used (10 mL); and
is the volume of juice used (10 mL); and 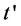 is the time (in min) needed for the pH to return to 7.7 after the addition of NaOH.
is the time (in min) needed for the pH to return to 7.7 after the addition of NaOH.
2.3.4. Total Phenolic Content
The total phenolic content of pineapple juice was determined according to a method adapted by Alothman, et al. [27] . A dilute pineapple juice (200 μL) was mixed with 1 mL of Folin-Ciocalteu’s (FC) phenol reagent. The FC phenol reagent was prediluted 10-fold with distilled water. After the pineapple juice-FC phenol reagent mixture was left at 30˚C ± 1˚C for 5 min, 800 µL of sodium carbonate (7.5%) was added. The solution was vortexed and allowed to stand for 2 h at room temperature. Then, the absorbance was measured at 765 nm with a spectrophotometer (UV-1600PC, Shanghai, China). The standard calibration curve was plotted using gallic acid (y = 0.043x − 0.051; R2 = 0.994). The mean of three readings was used and the results expressed as mg of Gallic Acid Equivalents (GAE)/100 mL of juice.
2.4. Microbial Analysis
All samples were analysed for mesophilic, thermophilic, psychrophilic bacteria (total bacterial counts), also for yeasts, moulds and coliforms during 8 weeks of storage at room temperature. Juice samples (1 mL) were decimal diluted serially with sterile 1 mg/mL peptone water and appropriate dilutions were poured on to the respective plates. Total bacterial counts were determined on plate count agar (PCA; Merck, Darmstadt, Germany) following incubation at 35˚C over 3 days for mesophilic bacteria, at 4˚C over 7 days for psychrophilic bacteria and 55˚C over 2 days for thermophilic bacteria. Yeasts and moulds were estimated on potato dextrose agar (Merck) and with incubation at (28˚C ± 1˚C) for 7 days. Coliforms were enumerated using Violet Red Bile Lactose Agar (Oxoid) at (36˚C ± 1˚C) for 48 hours. The number of individual colonies on plate was counted by visual observation in light. The actual CFU/100 mL of juice was then calculated using the following Equation (2):
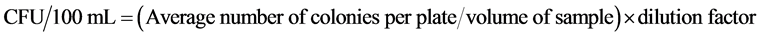 (2)
(2)
The microbial counts were expressed as log colony forming units (CFU)/100 mL. All microbiological analyses for each batch were conducted at least in triplicate for each experiment.
2.5. Statistical Analysis
All experiments were performed in triplicate. Data were expressed as mean ± standard deviation (SD). The Tukey’s test and one-way analysis of variance (ANOVA) used for multiple comparisons by the SPSS 17.0 (SPSS, Chicago, USA). Difference was considered to be statistically significant if P < 0.05.
3. Results and Discussions
3.1. Physico-Chemical Analyses of Pineapple Juice
3.1.1. Effect of Treatments on pH, Total Soluble Solid (TSS) and Titrable Acidity (TA)
Table 1 shows the changes in pH, titratable acidity (TA) and total soluble solids (TSS) in pineapple juice during two months of storage. The pH values for the
![]()
Table 1. Effect of treatments on pH, titrable acidity (TA) and total soluble solid (TSS).
Values are mean ± standard deviation of triplicates. Data in same column with different letters are significantly different (P < 0.05). Control: fresh pineapple juice without any treatment; MP: juice subjected to mild heat treatment at 65˚C for 15 min; US: juice subjected to ultrasound treatment for 15 min; UMP: ultrasound combined with mild heat pasteurization; TP: juice subjected to heat treatment at 80˚C for 15 min.
treated samples (MP, UMP and TP) varied between 4.10 and 3.95 suggesting the least significant differences (P > 0.05) among these values. The pH of the control and ultrasound treatment was found to decrease significantly (P < 0.05). Titrable acidity values evolved inversely compared to pH values during two months of storage. No significant change of TSS was observed for all samples except the control. In general, the acidity and the hydrogen ion concentration (indirectly the pH) in any fruit product vary due to different types of biochemical reactions such as hydrolysis, oxidation, fermentation, and decomposition.
Increased acidity and lowest TSS in control sample might be due to spoilage and fermentation, resulted to the conversion of sugar to acids, carbon dioxide or alcohol [28] . The major sugar in pineapple juice was sucrose (86%) and decreased during the fermentation. Carbohydrates are consumed during the fermentation due to the microbial growth. The pH decreased due to lactic acid production and sucrose hydrolysis occurred due to low pH values. These results were in agreement with the previous reports of Costa et al. [29] . Otherwise, in liquids, application of high power sonication creates the free radicals such as H+ and OH− radicals formed by the decomposition of water inside the cavities [21] [30] . That phenomenon might be responsible of the increased of pineapple juice acidity under the action of ultrasound treatment during the storage.
3.1.2. Determination of Browning Degree (BD)
Consumers consider product appearance to be the primary criterion for acceptance. Color has been considered to play a key role in fruit juice, preference and overall acceptance. It may even influence taste thresholds, sweetness perception and pleasantness. In this regard, it is important to bear in mind that for a given treatment, color maintenance should be given consideration to ensure that the outcome of the final product meets consumer choice and acceptability.
Browning degree of different pineapple juice samples are increased by increasing storage time (Figure 1(a) and Figure 1(b)).
![]() (a)
(a)![]() (b)
(b)
Figure 1. (a) and (b) Browning degree changes of pineapple juice at different types of treatment during storage at room temperature for 60 days. C: Control fresh pineapple juice without any treatment; MP: juice subjected to mild heat treatment at 65˚C for 15min; US: juice subjected to ultrasound treatment for 15 min; UMP: ultrasound combined with mild heat pasteurization; TP: juice subjected to heat treatment at 80˚C for 15 min.
During the first 15 days of storage, the thermal pasteurization and control following by ultrasound treatments, showed the lowest browning degree compared to other treatments. When the storage progressed to day 60, ultrasound sample showed significantly lower browning degree (P < 0.05). Spoilage of pineapple juice is not only encountered by microbial contamination but also by enzymatic degradation which is generally not accepted by the consumer [8] . The discoloring of fruit juice results from enzymatic browning, which is caused by the action of polyphenol oxidase (PPO) catalyzing oxidation of phenolic compounds [31] and pectin methylesterase (PME). PME is generally inactivated using conventional heat pasteurisation. The discoloration observed in thermal pasteurization sample will due to non-enzymatic browning through several biochemical reactions such as Maillard condensation, pigment destruction and caramelization of sugar [32] [33] . The lower browning observed in U treatment might be due to cavitation caused by sonication. Thus the mechanical effects and/or hydrogen peroxide (H2O2) formation in pineapple juice during sonication are responsible for the retardation of browning. Hydrogen peroxide (H2O2) has been demonstrated to exert an inhibitory effect on the browning to maintain whiteness of fruit and vegetable [34] . On the other hand, the lower browning observed in Control during the first 15 days of storage could be due to the carbon dioxide. In the presence of O2, the enzyme polyphenol oxydase (PPO) may oxidize these compounds to quinones which simultaneously polymerize into brown pigment [35] . Carbon dioxide inhibited the browning of pineapple juice and that PPO was competitively inhibited by CO2. This prevention of browning by CO2 probably was related to reduced PPO activity, since CO2 is a competitive inhibitor of PPO as demonstrated by Murr et al. [36] .
3.1.3. Pectin Methylesterase Activity
Pectin methylesterase (PME), an ubiquitous enzyme found in plants, hydrolyses pectin resulting in decreased cloud stability and reduced viscosity due to pectin chain degradation. The effects of ultrasound and heat treatments on PME activity of pineapple juice during storage are shown in Figure 2.
The lower PME activity observed with US treatment, during storage could be due to the mechanical damage of the pectin methylesterase protein structure during sonication. On the other hand, the lower pectin methylesterase activity observed with thermal pasteurization treatment could be due to the heat pasteurisation. Pectin methylesterase is generally inactivated using conventional heat pasteurisation.
Studies show that the PME activity of pineapple was inactivated when heating temperature increased from 20˚C to 90˚C [37] [38] . Chang et al. [39] and Goh et al. [40] reported that the PME activity in pineapple was reduced by heat pasteurisation.
3.1.4. Total Phenolic Content
The effects of different treatments during the storage time on the total phenolic content of pineapple juice are shown in Figure 3. All samples showed significant
![]()
Figure 2. Pectin methylesterase activity of pineapple juice during storage at room temperature for 60 days under different treatments.
![]()
Figure 3. Changes in total phenolic content in pineapple juice during storage at room temperature for 60 days under different treatments.
(P < 0.05) decrease on the total phenolic content during storage. There was a significant difference between control and treated samples at end of storage. Ultrasound followed by ultrasound combined with mild heat pasteurization treatments were effective in retaining the total phenolic content of pineapple juice as compared to the thermal treatment or the untreated juice sample at room temperature during 60 days of storage. The results confirmed that the application of ultrasound treatment preserved the total phenolic level of fruit juices during the storage. Similar results have been reported for the studies of different fruit and vegetable juices [41] . The changes in the levels of phenolic compounds indicate the deteriorating quality of fruit products due to browning, formation of hazes and sediments [42] . The decrease in the phenolic content of the samples during storage usually has effects on the color parameters. The lower total phenolic content observed with MP and TP treatments, during storage could be due to thermal pasteurization. According to Goh et al. 2012, [40] , conventional heat pasteurisation is able to inactivate pectin methylesterase in pineapple juice, it compromises the heat sensitive phenolic compounds. Thus, at a higher temperature like 70˚C, thermal degradation of total phenolic was faster resulting to a lower amount of bioactive total phenolic in the sample [43] . Our results showed direct correlation between phenolic compounds and browning degree.
3.2. Microbial Analysis
Organisms which are usually responsible for spoilage of fruit juice include gram-negative, psychrotrophic bacteria, yeast and moulds. Figures 4(a)-(d) shows mesophilic, psychrophilic, yeasts and moulds counts of pineapple juice treated with US, MP, UMP, TP and control stored for a period of 60 days at room temperature.
Gradual growth of all microorganisms was seen during storage in all samples. However, some treatments retarded the microbial growth more than others. Generally, yeasts and moulds were present in relatively lower numbers during the storage. Coliforms and thermophilic were not founded in any samples. The highest amount of microorganisms was observed in control samples. Thermal pasteurization (TP) followed by ultrasound combined with mild heat pasteurization and ultrasound treatment, in increasing order, were found to be effective in delaying microbial growth in pineapple juice. Thermal pasteurization treatments were effective in retaining the microbial growth in pineapple juice during storage at room temperature. Unfortunately, due to the high temperature used in the process, the nutritional and sensory properties of the pasteurized juice were somewhat altered. Many researchers have also demonstrated that heating is the most widely accepted technique used for inactivating microorganisms in food, however there can be a significant change in the functional properties and contents of food, which tends to reduce the product quality and freshness [41] [43] [44] [45] . The lowest amounts of microorganisms found in UMP and US treated samples might be due to ultrasound process that creates the cavitation caused by the changes in pressure responsible for the destruction of bacteria. The mechanism of microbial destruction is mainly due to thinning of cell membranes, localized heating and production of free radicals [46] [47] . These observations are in agreement with those authors reported that ultrasound can reduce the microbial levels on strawberry [48] and plum fruit [49] . The results of a research carried out by Dolatowski et al. [50] proved that ultrasound processing is having a significant influence on microbiological contamination of meat.
4. Conclusion
Compared to the untreated (control) samples, treated samples had significantly (P < 0.05) longer shelf-life. The ultrasound and combination of non-thermal methods are a new approach for preservation of fruit juices that can enhance the microbiological safety while having lower impact on the organoleptic and nutrient properties of juices in comparison with the thermal processing.
Acknowledgements
The authors would like to thank Dr. Abdou Madjid O. Amoussa, for technical assistance.