Investigation of Ca and Mg Removal Capability of Cactus Powder from Hard Water ()

Subject Area: Analytical Chemistry

1. Introduction
1.1. Background
Hard water is not a health hazard, but dealing with hard water causing spot and brightness on cloth. Water that contains bicarbonates, chlorides and sulphates of calcium and magnesium metal is hard in nature [1] . Hardness of given water is a measure of the total concentration of Ca and Mg ions since dissolved calcium and magnesium are the two most common minerals that make the water “hard” [1] [2] . Hardness in water is not caused by a single substance but also by a variety of dissolved polyvalent metallic ions such as aluminium (Al3+), barium (Ba2+), manganese (Mn2+), iron (Fe2+) and zinc (Zn2+) [1] - [3] . But these ions typically make up only a very small fraction of total hardness [2] .
1.2. Sources of Hard Water
The nature of hardness or softness of water varies from place to place. This reflects the nature of the geological properties of the area where the water has been contacted in [3] . The principal natural sources of hardness in water are the dissolved polyvalent metallic ions from sedimentary rocks, seepage and runoff from soils [4]. Calcium and magnesium are the two principal metal ions present in many sedimentary rocks such as limestone and chalk [1] [3] . They are also common essential mineral constituents of food [1] . As mentioned before, a minor contribution to the total hardness of water is also caused by other polyvalent ions, such as aluminum, barium, iron, manganese and zinc [1] . Therefore, hardness of given water is caused by many cationic and anionic species.
1.3. Effect of Hard Water
Hardness in drinking water is defined as those minerals dissolved in water having positive and negative electrical charges [2] [4] [5] . According to National Research Council (NRC) and World Health Organization (WHO) reports, hard water doesn’t have health impacts [2] . But, the amount of hardness minerals in given water affects the amount of soap and detergent that is necessary for cleaning [4] . When hard water combines with soap, it gets a precipitate to form Ca and Mg salts [3] [5] . This prevents the formation lather with soap and it requires considerable amounts of soaps to produce lather [4] [6] . Hard water also produces a conspicuous deposit of precipitates such as insoluble metals, soaps or salts in containers, including “bathtub ring” [5] .
Many people are facing challenges in the area of laundering and dishwashing of bathing and personal grooming [6] . They lose much time and many soaps to clean their cloths since soaps used in hard water combine with the minerals to form sticky soap curd [4] - [6] . This causes graying of white fabric on cloth and the loss of brightness in colors due to incomplete soil removal during laundry work [5] .
Bathing with soaps in hard water causes a film of sticky soap curd on the skin which prevents soil and bacteria from being removed [3] [5] . This soap curd interferes with the return of skin to normal condition, is slightly acid in nature and may lead to irritation and itching of the skin [2] . Moreover, hard water causes soap curd on hair which makes the hair dull, lifeless and gray [1] .
Besides, hard water also causes a film on glass shower doors, shower walls, bathtubs, sinks, faucets and dishes, which may be spotted when they dry [4] [6] . Furthermore, hard water deposits build in thickness that act as insulation, reducing the efficiency of heat transfer and conduction [3] .
As described above, “hard water” contains high amounts of dissolved calcium and magnesium ions [1] - [3] . The presence or absence of these hardness minerals in drinking water is not known to pretend a health risk to users, rather poor soap and detergent performance in laundry [1] .
Magnesium (Mg): It presents as exchangeable magnesium ion as cation-exchange complex and as solution in soluble Mg2+ ions [7] . Mg is used as a cofactor of many enzymes involved in carbohydrate and fat metabolism, protein synthesis, photosynthesis, and RNA and DNA synthesis [4] [7] . It is an active component of several enzyme systems in thymine pyrophosphate, and oxidative phosphorylation for activation of phosphate-transferring enzymes like myokinase, diphosphopyridine, nucleotide kinase and creatine kinase [6] [7] . It activates pyruvic acid carboxylase and pyruvic acid oxidase in the citric acid cycle [7] . It also acts as the main source of magnesium lactate which inhibits histidine decarboxylase and prevents the formation of histamine from the amino acid and histadine [7] .
Excessive intakes of Mg lead to hypomagnesaemia while its deficiency enhances diabetes mellitus and gastrointestinal tract abnormalities [2] [3] [5] . Drinking-water in which both magnesium and sulfate are present at high concentration can have a laxative effect [3] [5] [7] . Besides, high amounts of Mg in water prevent lather formation of water with soap. This leads to the use of high amounts of soap which causes loss of economy.
Calcium (Ca): Calcium is a divalent alkaline earth metal and the 5th most plentiful element in the earth’s crust [7] . It occurs in plant tissues as free Ca2+ and as combined form such as carboxylic, phosphorylic, phenolic hydroxyl groups, oxalates, carbonates and phosphates [2] [6] . It is essential to health in the osteoarticular system, in development and maintenance of the skeleton and in cardio-muscular systems [2] [7] . It is also used in blood coagulation, muscle contraction and nerve impulse transmission, in the conversion of prothrombin to thrombin and in milk clotting [7] . It is important to regulate blood pressure and kidney function and reduce blood cholesterol level [7] .
A high calcium intake leads to urinary stone formation, rickets and osteoporosis; it inhibits the intestinal absorption of iron, zinc and other essential minerals [3] [7] . When calcium is absorbed in excess of need, the excess is excreted by the kidney in healthy people who do not have renal impairment [7] . Calcium interacts with iron, zinc, magnesium and phosphorus within the intestine, thereby reducing the absorption of these minerals. Moreover, the presence of high amounts of Ca ion in water also causes hard water [5] [7] . This hard water consumes high amounts of soaps and detergents in bathing and laundering.
Currently, many methods have been adopted to treat hard water like reverse osmosis, ion exchange and others, but these methods are costly and not available plentifully. Therefore, this study is designed to investigate the capability of cactus powder to remove Ca and Mg metals from hard water using Flame Atomic Absorption Spectrometer (FAAS).
2. Methodology and Material
2.1. Description of Study Area
The present study is conducted in Adigrat University, in northern region of Ethiopia. Adigratis a city and separate woreda that found in Tigray Region of Ethiopia. It is located in the eastern zone around 900 km far from Addis Ababa which is capital city of Ethiopia with longitude 14˚16'N 39˚27'E coordinates and latitude 14˚16'N 39˚27'E with an elevation of 2457 meters above sea level. Adigrat is endowed with cactus plant which is harvested once in a year. The largest pharmaceutical manufacturing plant in Ethiopia, “Addis Pharmaceuticals Factory SC”, is also located in Adigrat.
2.2. Equipment and Instruments
Atomic absorption spectroscopy (buck scientific, model 210VGP FAAS) equipped with deuterium arc back ground correctors and hollow cathode lamps with air-acetylene flame was used for the investigation of Ca and Mg metals from hard water.
2.3. Reagent and Chemical
Stock standard solutions containing 1000 ppm of the metals Ca and Mg were used for preparation of standards. Deionized water was used for rinsing of the glassware, sample bottles and for dilution of the sample solution.
2.4. Collection of Plant Samples
Mature healthy and fresh leaves of cactus were collected from side of Adigrat town. The collected leaves were washed with distilled water and the leaves were cut in to small pieces. The tip egged and their thick epidermis was removed. They were dried at 70˚C - 90˚C in electric oven. The dried plant materials were ground into powder form using pestle and mortar.
2.5. Procedure
Four ppm of a known Concentration of Ca metal and six ppm of Mg were added into six 100 mL round flasks. Different dosages of cactus (0.5, 1.5, 2.5, 3.5, 4.5 g) were added and were filled with deionised water up to the mark of the flask. The solution in flasks were homogenized and allowed to settle for a few time. The absorbance was measured using FAAS. The absorbance was compared in the present and in the absent of cactus powder. The percentage removal (%) of Ca and Mg metals by cactus was calculated using the equations [8] :
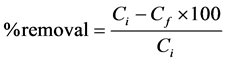 where, Ci and Cf are the Concentrations of the Ca and Mg metals in the absent of cactus and in the present of cactus, respectively.
where, Ci and Cf are the Concentrations of the Ca and Mg metals in the absent of cactus and in the present of cactus, respectively.
The percentage removal (%) of Ca and Mg metals at various contact time was also calculated using as:
 where, Cto and Ct are the Concentrations of the Ca and Mg metals at initial and
where, Cto and Ct are the Concentrations of the Ca and Mg metals at initial and
final time, respectively.
2.6. Statistical Analysis
After the results were obtained and recorded, the mean, standard deviation, regression and correlation factors, Concentration and others were performed using data analysis packages such as Microsoft Excel 2007 and originlab 8.1. Mahts type software also uses to write mathematical equations. All measurements were done in triplicate and the results were reported as average values ± SD.
3. Result and Discussion
3.1. Instrument Calibration
The calibration curves were plotted as a function of absorbance versus Concentration of the standard solution. In this study two calibration curves were plotted for Ca and Mg metal as shown in Figure 1.
3.2. Effect of Cactus Dosage on Removal of Ca and Mg Metal from Hard Water
Calcium and magnesium ions are the main factor that contributes the hardness of water. These ions can form a precipitate with soap which is causing the buildup of soap scum [1] . This is due to the soap molecules are being precipitated by the Ca2+ and Mg2+ ions; there is less soap available to form lather [1] . This takes a considerable
![]()
![]()
Figure 1. The calibration graphs of Ca and Mg metal standard solution.
amount of soap to form lather which leads loss of economy and time. Besides, taking of a shower in hard water can be very frustrating and cause itching of skins [3] [5] .
Additionally, hard water blocks boiler scale [2] - [4] . When hard water comes into contact with dissolved carbonates, a precipitate of insoluble calcium carbonate can form and build up on inside of water pipes which completely blocked the pipes [4] . By illumination the above impact of hard water, this study is focused to examine the hard water removal efficiency of cactus powder using FAAS.
As shown in Figure 2 and Table 1, the Concentration of Ca is decreased from 4.967 ppm to 1.033 ppm as the dose of cactus powder increased from 0.0 g to 4.5 g. The Concentration of Ca metal from hard water decreased significantly from 4.967 to 0.933 ppm as the dose of cactus powder increased from 0.0 g to 2.5 g. But, the Concentration of Ca remains constant beyond 2.5 g dose of cactus powder. This is due to the fact that the equilibration of the active sites of cactus powder and the trapped Ca metal. In similar way, the Concentration of Mg metal is decreased from 5.965 ppm to 1.90 ppm as the dose of cactus powder increased from 0.0 g to 4.5 g. But the slight increment (almost constant) value of the Concentration of Mg metal above 2.5 g of cactus powder is due to equilibration of the ambushed Mg metal in the active sites of cactus powder. This result reveals that, 2.5 g powder of cactus powder is enough to bind the Mg metal ion from the given hard water.
The Concentration of Mg metal increased 5.965 ppm to 6.700 ppm when 0.5 g of cactus powder is added. This might be due to the fact that, adding of a small amount of cactus dose (0.5 g) isn’t sufficient to bind the metal ion from a given hard water since the cactus powder is dissolved in the hard water and in turn contribute the increment of Mg metal. Therefore, optimize dose of cactus powder is too important to remove Ca and Mg metal from hard water.
In general, the Concentration of both Mg and Ca metal are decreased as the dose of cactus is increased. The Concentration of Mg was 5.965 ppm in the absent of cactus powder, but it is 6.700 ppm at 0.5 g, 2.967 ppm at 1.5 g, 1.833 ppm at 2.5 g, 2.200 ppm at 3.5 g and 1.900 ppm at 4.5 g of cactus powder. The slight increment and almost constant Concentration of Mg metal exceeding 2.5 g cactus powder is due to the equilibration of Mg ion in the active sites of cactus powder. The other possible reason may be further addition of cactus powder beyond the optimum dose results re-dissociation of the trapped metal ions from the active sites of cactus powder.
In parallel way, the Concentration of Ca was 4.967 ppm in the absent of cactus powder, 3.067 ppm at 0.5 g, 1.667 ppm at 1.5 g, 0.933 ppm at 2.5 g, 0.967 ppm at 3.5 g and 1.033 ppm at 4.5 g of cactus powder (Figure 2 and Table 1).
As it has been seen in Figure 3, the percentage removal of both Mg and Ca metal from hard water is increased as the dose of cactus powder increased. The hard water (Mg) removal capability of cactus powder is increased from 0.00% to 68.2% as the cactus powder increased from 0.0 g to 4.5 g. In the same way, the percentage removal of hard water (Ca) is increased from 0.00% to 79.2% as the dose of cactus powder increased from 0.0 g to 4.5 g. The slight decrement of percentage removal for both Ca and Mg metal beyond 2.5 g cactus powder might be due to the fact that re-dissociation of the trapped metal ions from hard water. The other probable reason may be the exhausting of the active site of cactus powder that will need to be regenerated continuously. Thus, in high adsorbent dose, the adsorption capacity is reduced due to overlapping of adsorption sites on adsorbent surface [1] [5] .
![]()
Figure 2. Effect of cactus dose on decrement of Ca and Mg metal from hard water at initial concentration of 4 ppm and 6 ppm, respectively.
![]()
Figure 3. Percentage removal capability of cactus powder Ca and Mg metal from hard water treatment.
![]()
Table 1. Effects of cactus dose on removal of Ca (4 ppm) and Mg (6 ppm) metal from hard water.
3.3. Effect of Contact Time on Removal of Ca and Mg by Cactus Powder from Hard Water
As it can be seen from Figure 4 and Table 2, the removal capability of cactus powder for Ca and Mg metal ions are increased with contact time. The effect of contact time on removal of Ca and Mg metal from hard water by cactus powder can be determined by phasing in different contact time. The percentage removal of Ca metal increased with contact time as 27.89% at 1 hr, 38.69% at 2 hr, 53.27% at 3 hr, 71.11% at 4 hr and 71.53% at 5 hr.
In the same way, the percentage removal of Mg metal increased with contact time as 16.00% at 1hr, 32.84% at 2 hr, 48.00% at 3 hr, 61.00% at 4 hr and 57.00% at 5 hr. As the result indicates that the percentage removal of Mg metal is decreased slightly beyond 4hr. This might be due to the re-dissociation of the trapped metal after end point of the adsorbed metal.
In general, the percentage removal of Ca and Mg metal ions from hard water is increased with contact time. This is the reason why as the contact time increased there is formation of stable complexes between the Ca and Mg metal ions with the active site of the cactus powder (Figure 4). Hence, the removal of Ca and Mg metal by cactus powder increased with the contact time (Figure 3 and Table 2).
Cactus powder has the capability to remove metal ions (Ca and Mg) from hard water due to the accessibility of carboxyl, carbonyl and hydroxyl functional group as studied by various researchers Balaria and Schiewer (2008, 2009). Spectroscopic studies indicate the involvement of CO (carboxyl and carbonyl) and OH (hydroxyl) functional groups of the cactus powder leads in hydrogen bonding and donor-acceptor interactions between Ca and Mg metal ions to form coordinated compounds [9] [10] . Therefore, cactus powder has the capability to trap metal ions from hard water by it pore sites/ functional group (Figure 5).
As shown in Figure 5, cactus powder has ability to bind metal ions from hard water via it active sites. Hence, cactus is crucial in removing of metal ion from a given samples.
4. Conclusion
The result revealed that the concentration of both Mg and Ca metals decreased as the dose of cactus increased. The percentage removal of both Mg and Ca metals from hard water increased as the dose of cactus powder
![]()
Figure 4. Effect of contact time on Ca and Mg ions removal using 2 g cactus powder at initial 4 ppm of Ca and Mg at different time (hour).
![]()
![]()
Figure 5. Mechanist trapping of Ca and Mg metals by cactus powder.
![]()
Table 2. Effects of contact on removal of Ca (4 ppm) and Mg (6 ppm) metal from hard water.
increased. The percentage removal of cactus powder increased from 0.00% to 68.2% for Mg and from 0.00% to 79.2% for Ca as the cactus powder increased from 0.0 g to 4.5 g, respectively. The percentage removal of Ca and Mg ions from hard water increased with contact time. In general, cactus powder has the capability to remove metal ions such as Mg2+ and Ca2+ from hard water. Thus, the removal ability of cactus powder increases with dose of cactus powder and contact time. Thus, this study invites the societies, researchers and people who work in launder to use cactus powder to relive the effect of hard water.
Acknowledgements
Researchers want to express their thanks to the college of Natural and computational science, department of chemistry, Arba Minch University and Mekelle University for providing laboratory facilities. They extend their pleasure to Adigrat University, Ethiopia, for financial supports for this study.
NOTES

*Corresponding author.