Gentamicin Renal Excretion in Rats: Probing Strategies to Mitigate Drug-Induced Nephrotoxicity ()
Received 23 November 2015; accepted 18 January 2016; published 21 January 2016

1. Introduction
Aminoglycosides have been used for many decades to treat serious infections [1] . Gentamicin is the most commonly prescribed aminoglycoside, in part due to its low resistance levels and low cost [1] . Gentamicin is active against most strains of gram negative and some gram positive bacteria, with relatively low incidences of tolerance [2] . The therapeutic use of gentamicin has generally been restricted to life threatening infections, as the compound is nephrotoxic at therapeutic doses [3] [4] . The incidence of aminoglycoside nephrotoxicity in patients is approximately 25% [5] . However, because of the emergence of multi-drug resistance of bacteria to less toxic antimicrobial medications, clinicians are forced to consider aminoglycoside therapy for nosocomial infections in hospitalized patients and enterococcal endocarditis [6] . Thus, gentamicin is frequently used as a first or second choice drug in the clinic [4] . Given its continued use in drug therapy, considerable research has been aimed at developing approaches to reduce aminoglycoside toxicity in patients.
Despite significant research in this field, the molecular mechanism associated with gentamicin nephrotoxicity is not completely understood. Gentamicin is a hydrophilic cationic compound that does not readily penetrate cell membranes [7] . In vivo, approximately 90% of a gentamicin dose is recovered in the urine [8] . However, the drug selectively accumulates in the proximal tubule at concentrations much higher than those measured in plasma, and with a longer half-life in the tubular cell [1] . Once inside the kidney cell, gentamicin concentrates in lysosomes, endosomes and within the Golgi complex [9] . As drug concentrations rise, gentamicin empties into the cytosol, where it induces apoptosis and necrosis and inhibits various kidney membrane transporters leading to altered tubular reabsorption and reduced cellular viability [5] .
The mechanisms of gentamicin uptake and accumulation in the kidney have been the subject of numerous published reports [1] [7] [10] - [17] . Elucidation of these pathways can provide insight into the mechanism of aminoglycoside nephrotoxicity. A study comparing renal accumulation in filtering and non-filtering kidneys demonstrated that gentamicin uptake proceeded via reabsorption across the luminal membrane of the proximal tubular cell [18] . Subsequently, it was established that aminoglycoside uptake involves absorptive endocytosis mediated by megalin [10] , although other reports suggest that other transport pathways may be involved that do not require endocytosis [7] [17] . Thus, it appears that gentamicin uptake and accumulation may involve multiple processes.
Two general strategies have been proposed to protect against aminoglycoside nephrotoxicity [4] [9] . The first strategy involves reducing drug accumulation in the kidney. Administering gentamicin as a single daily dose has been suggested to be less nephrotoxic, due to saturation of luminal uptake resulting in reduced concentrations in the kidney [1] [5] [9] [19] . Alternatively, inhibitions of megalin-mediated endocytosis or other transport pathways through direct competition or other approaches have also been tested [10] [20] . A second strategy aims to reduce toxicity through co-administration of renoprotective compounds, including antioxidants [21] - [23] .
The objective of this investigation was to explore alternative methods to reduce gentamicin uptake into the proximal tubule cell, the critical step leading to aminoglycoside nephrotoxicity. Experiments were performed using the isolated perfused rat kidney (IPRK) model. The IPRK can be used to study numerous aspects of renal drug disposition. Applications of the model include elucidating renal excretion mechanisms, screening for potential drug-drug interactions, and assessing renal drug metabolism [24] . Thus, it is a useful preclinical tool for the current investigation.
The specific aims of the research were: 1) to assess the dose-linearity of gentamicin excretion over a range of clinically-relevant concentrations; and 2) to probe potential strategies for reducing the kidney accumulation of gentamicin, including urinary alkalization and transporter-inhibition. Urinary alkalization was induced through administration of sodium bicarbonate (NaHCO3), and the effect of increased urine pH on gentamicin excretion was determined. Transport inhibition studies were carried out using cimetidine, a known inhibitor of organic cation transport in the kidney [25] .
2. Material and Methods
2.1. Chemicals
Fraction V bovine serum albumin (molecular weight range 69,000 to 78,000 D), dextran (clinical grade, molecular weight range 60,000 to 90,000 D), inulin (from chicory root), amino acids, potassium chloride, sodium chloride, sodium bicarbonate, magnesium sulfate, calcium chloride, glucose, sodium bicarbonate (NaHCO3), cimetidine and gentamicin (sulfate salt) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium hydroxide and pH calibration standards were obtained from VWR Scientific Products (West Chester, PA). Solvents used for HPLC were obtained from J & H Berge Co. (Plainfield, NJ). Amicon Centrifree YM-30 (molecular weight cut off 30 K) centrifugal filter devices were obtained from Millipore Corporation (Billerica, MA).
2.2. Animals
Male Sprague Dawley rats (250 - 350 g) were used for perfusion experiments. The rats were purchased from Harlan Laboratories (Indianapolis, IN). All rats were caged in stainless steel cages and fed standard chow and water ad libitum. The Institutional Animal Care and Usage Committee (IACUC) of Long Island University approved the experimental protocol for this investigation.
2.3. Isolated Perfused Rat Kidney Preparation
IPRK experiments were carried out as described previously [24] [26] . The surgical procedure involved cannulation of the right kidney via the superior mesenteric artery, using a technique that maintained a continuous flow of perfusate to the kidney, thereby decreasing the possibility of ischemia during the isolation of the kidney [26] . The perfusate consisted of Krebs-Henseleit buffer (pH 7.4) containing BSA (4%), dextran (1.67%), glucose (0.1%), inulin (GFR marker, 0.06%) and amino acids.
Anesthesia was induced with an intraperitoneal injection of sodium pentobarbital (40 mg/kg). A midline incision was made and the renal segment of the aorta exposed. A ligature was passed under the right renal artery close to the aorta, and distal and proximal ligatures placed around the superior mesenteric artery. The right ureter was catheterized with polyethylene (PE-10) tubing in order to facilitate urine collection. A cannula was then threaded through the mesenteric artery, across the aorta, and into the right renal artery in situ. The ligatures were tied, securing the cannula in place. The right kidney was then excised from the animal, trimmed of adhering tissue and transferred to the in vitro recirculating perfusion apparatus.
2.4. Treatment Groups
Control perfusions were conducted to test system suitability and viability of the study model, and to assess effects of treatments on kidney function. The renal excretion of gentamicin was determined over a range of doses targeting initial perfusate concentrations between 5 and 40 µg/ml. A total of four doses were studied (5, 10, 20 and 40 µg/ml). Three additional study groups were carried out to determine the effect of urinary alkalization and/or transport inhibition on gentamicin excretion: 1) Gentamicin (10 µg/ml) co-administered with NaHCO3 (0.25 mM); 2) Gentamicin (10 µg/ml) co-administered with cimetidine (2 mM) (OCT inhibitor); and 3) Gentamicin co-administered with NaCO3 (0.25 mM) and cimetidine (2 mM). A total of five perfusion experiments were conducted for each study group.
2.5. Experimental Design
Each IPRK experiment was conducted over a 2-hour period. Once the kidney was placed in the perfusion apparatus, a stabilization period (10 minutes) preceded any pharmacokinetic experimentation. For the dose linearity experiments, a bolus dose of vehicle (KHS buffer, dose linearity perfusions), NaHCO3 or cimetidine (or both) was administered following the stabilization period. After a ten minute distribution phase, gentamicin was then added to the perfusion reservoir as a bolus dose. The time was denoted as time zero for pharmacokinetic calculations.
A perfusate sample was collected 5 minutes post-dose and every 10 minutes thereafter for a total of 100 minutes. Urine was collected in 10-minute intervals throughout the experiment. After each collection interval, urine volume was determined gravimetrically and pH was measured. Perfusate and urine samples were analyzed for electrolytes (sodium, chloride) and glucose using a Beckman Synchron CX-3 Clinical Chemistry Analyzer (Beckman-Coulter, Brea CA). Inulin (GFR marker) was measured using colorimetric method [27] . Urine and perfusate samples were stored at −20˚C prior to the analysis of gentamicin.
During the course of the experiment, the perfusion pressure was maintained at 100 ± 10 mm Hg by adjusting the perfusate flow rate as needed. The volume of the recirculating perfusate (80 mL) was maintained constant by addition of the replenishing solution that was prepared with a 1:1 dilution of the perfusate and deionized water. At the end of the experiment, the kidney was removed, blotted dry, and weighed. Kidney function and viability was assessed by the following parameters: GFR, reabsorption of electrolytes and glucose, urine flow rate, and urine pH.
2.6. Perfusate Binding of Gentamicin
Perfusate binding of gentamicin was measured by ultrafiltration. Four concentrations of gentamicin were studied: 1, 5, 10, and 40 µg/ml. Perfusate samples were incubated at 37˚C under constant stirring for 60 min to ensure binding equilibrium. After incubation, an aliquot of sample was collected for the determination of total drug perfusate concentration. A second aliquot (1 ml) was added to an Amicon Centrifree Micropartition System (Millipore Corporation, Bedford, MA) and the device was centrifuged at 1500 × g for 15 min. After centrifugation, the resulting ultrafiltrate was stored at −20˚C for subsequent determination of free drug concentration. Preliminary studies determined that the drug binding to the device was negligible. All studies were performed in triplicate. The fraction of gentamicin unbound in perfusate (fu) was calculated as the ratio of unbound and total concentration.
Further studies were performed to evaluate nonspecific binding to IPRK apparatus for all gentamicin doses used in current investigation. These studies were performed using KHS buffer and gentamicin was added as a bolus dose. Samples were collected 5 min post-dose and every 10 min thereafter over 2 hour.
2.7. HPLC Analysis
Structurally, gentamicin does not possess ultraviolet light absorbing properties, and therefore the compound cannot be analyzed directly by UV or fluorescence detection. Consequently, HPLC methods for gentamicin typically include pre-column or post-column derivatization. There are a number of published methods for gentamicin involving various derivatizing agents [28] - [33] , which were used by other investigators. In the present investigation new HPLC method was developed and validated [34] . The method utilized in this investigation involves pre-column derivatization of gentamicin with O-phthalaldehyde (OPA) and 2-mercaptoethanol (MPE). In contrast to published gentamicin HPLC assays, 1) this assay requires a low sample volume; 2) involves simple and less time-consuming derivatization process, 3) the derivative produced is more stable (up to 4 hours) at room temperature and 4) the sensitivity of assay is higher, thus it can be used to measure gentamicin concentrations at therapeutic doses.
Gentamicin was quantified using an HPLC method with fluorescence detection. Test samples (perfusate, urine, ultrafiltrate) were treated with 1% ZnSO4 (1:1 ratio) to precipitate proteins. Derivatizing agents were spiked directly into the resulting sample extract. Analyte separation was accomplished using a Hypersil ODS C18 column (150 mm × 4.6 mm, 5 um particle size). The mobile phase consisted of 0.02 M sodium heptanesulfonic acid in methanol-glacial acetic acid-water (70:5:25) (pH 3.4). Analysis was conducted at ambient temperature at a constant flow rate of 2.0 mL/min using isocratic elution. Gentamicin was detected at an excitation maximum of 360 nm and emission maximum of 430 nm.
A standard addition method [35] was used to increase the sensitivity of the assay in perfusate. The method involved the addition of known quantities of gentamicin to multiple aliquots of a perfusate sample. The standard samples were analyzed, and the concentration of gentamicin in the sample was determined from a plot of detector response (peak height) versus gentamicin concentration.
2.8. Data Analysis
For each urine collection period, the renal clearance of inulin (GFR) was calculated using the following equation:
 (1)
(1)
where UFR represents the urine flow rate, Uinulin is the concentration of inulin in urine, and Pinulin is the perfusate concentration of inulin sampled at the midpoint of the urine collection interval.
The following equation was used to calculate clearance (Cl) of gentamicin in the IPRK:
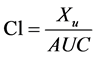 (2)
(2)
Xu refers to the cumulative urinary excretion of gentamicin and AUC is the area under the curve over the duration of the perfusion experiment (100 minutes). AUC was estimated using the trapezoidal rule.
Renal excretion ratio (XR) is a parameter used to determine net mechanisms of renal excretion. XR was calculated as follows:
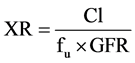 (3)
(3)
Kidney accumulation of gentamicin was estimated using mass balance analysis; that is, as the difference between the administered dose and the total amount of drug remaining in the perfusate and recovered in the urine at the end of the perfusion experiment.
2.9. Statistical Analysis
Estimates of IPRK viability parameters for control and drug treatment groups were compared using analysis of variance (ANOVA). Post-hoc analysis (Dunnett’s Test) was utilized to identify study groups that differed from control perfusions in terms of viability criteria. Consequently, alterations in kidney functions induced by drug administration or NaHCO3 were determined. Likewise, mean values for gentamicin pharmacokinetic parameters were compared using ANOVA. Post-hoc analysis (Tukey HSD) was once again used to identify differences am- ong the various treatment groups in an effort to identify differences in gentamicin excretion as a function of dose or co-administration of NaHCO3 and/or cimetidine.
3. Results
3.1. Quantification of Gentamicin
In current investigation, pre-column derivatization with 2-mercaptoethanol (MPE) with OPA was used to improve assay sensitivity. The derivative was found to be stable in the mobile phase for up to 4 hours at room temperature. The variability of the method with regard to reproducibility, accuracy, and precision was within acceptable limits (Table S1, Table S2). Nearly complete recovery of the drug from the matrix was obtained. Limit of detection (LOQ) of the HPLC assay method was high (10 μg/ml). In order to measure gentamicin perfusate concentration in the clinically relevant range, a standard addition method was employed. The method involved the addition of known quantities of gentamicin to multiple aliquots of a plasma sample. The standard samples were analyzed, and the concentration of gentamicin in the sample was determined from a plot of detector response (peak height) versus gentamicin concentration. This approach lowered the LOQ of the method in perfusate to 1 μg/ml. Gentamicin was stable in all matrices for 24 hr at room temperature and up to 1 month at −20˚C.
3.2. Gentamicin Perfusate Binding
Gentamicin binding in IPRK perfusate was constant over the range of concentrations tested (5 - 40 µg/ml). Bin- ding was estimated as 12% ± 0.9% (fu = 0.88). In a separate experiment, gentamicin was not found to undergo non-specific binding of gentamicin to the IPRK apparatus.
3.3. Dose Linearity Assessment
A summary of the parameters used to assess IPRK suitability is presented in Table 1. Control (drug-naive) studies were performed to establish the viability of the preparation and to assess the effect of varying doses of gentamicin on kidney function. The data presented in the table are consistent with published values [24] [26] [36] [37] . There were no significant differences in parameter estimates among any of the treatment groups compared to control (p-value > 0.05), indicating that that kidney function was well preserved in the presence of varying doses of gentamicin over the duration of the IPRK experiments.
Estimates for gentamicin renal excretion parameters are provided in Table 2. To correct for inter-kidney variability, GFR and clearance estimates were normalized for kidney weight. Although the drug appeared to exhibit dose-linearity with respect to AUC, the data indicate a non-linear renal excretion profile with increasing dose. Gentamicin excretion ratio (XR) was less than one across all groups, consisted with net reabsorption by the
![]()
Table 1. IPRK viability parameters: dose proportionality experiments.
aData reported as mean (standard deviation) representing five perfusion (n = 5) per treatment group. Ten excretion periods were analyzed for each perfusion experiment; bGlomerular filtration rate, normalizes per kidney weight; cFractional reabsorption of glucose; dFractional reabsorption of sodium; eFractional reabsorption of chloride.
![]()
Table 2. Gentamicin renal excretion parameters in IPRK: dose proportionality experiments.
aGlomerular filtration rate, normalized for kidney weight; bCalculated as the ratio of cumulative urinary excretion and AUC for duration of IPRK experiment (data are corrected for kidney weight); cCalculated are ratio of clearance (clearance/(fu × GFR)); dEstimated from mass balance analysis at end of experiment (dose-cumulative excretion-amount remaining in perfusate); eArea under the curve was estimated using trapezoidal rule. Estimates were divided by gentamicin dose; *Indicates values that are significantly different from all other treatment groups; **Indicates values that are significantly different from 20 µg/ml and 40 µg/ml treatment groups; ***Indicates values that are significantly different from 10 µg/ml treatment group.
kidney. Likewise, clearance and cumulative excretion decreased with increased gentamicin dose. Gentamicin kidney accumulation, estimated by mass balance, ranged from ~20% - 30% among the treatment groups.
3.4. Interaction Studies
A second set of perfused experiments was carried out to assess whether the excretion profile and kidney accumulation of gentamicin could be altered through changes in pH or transport inhibition. Accordingly, gentamicin was co-perfused with NaHCO3 (to urine pH) and/or cimetidine (inhibitor of basolateral transport). As presented in the Table 3, there were no significant differences in perfusion flow rate, urine flow rate, GFR and FRChloride among all study groups. Urine pH was significantly higher in NaHCO3-treated perfusions, which is relevant to the study design of those experiments. Although differences in glucose and sodium reabsorption were noted treatments, parameter estimates within acceptable ranges [24] .
A plot of gentamicin perfusate concentrations vs. time is presented in Figure 1. The graph shows the cimetidine co-administration decreased the gentamicin elimination from the IPRK, whereas perfusate levels decreased more rapidly in the presence of NaHCO3. As noted in Figure 2, gentamicin urinary excretion was increased in the presence of NaHCO3.
Renal excretion parameters for these interaction experiments are summarized in Table 4. The data illustrate that NaHCO3 co-perfusion increased gentamicin elimination in the IPRK, as clearance, cumulative excretion and XR were all significantly increased compared to experiments with gentamicin alone. Whereas gentamicin clear- ance was apparently not impacted by cimetidine administration, kidney accumulation was significantly reduced.
![]()
Table 3. IPRK viability parameters: interaction experiments.
aGFR normalized per kidney weight; bFraction reabsorption of glucose; cFraction reabsorption of sodium; dFraction reabsorption of chloride; *Indicates values that are significantly different from control, gentamicin and gentamicin + cimetidine treatment groups; **Indicates values that are significantly different from all other treatment groups.
![]()
Figure 1. Plot of gentamicin perfusate concentration vs. time in the IPRK: Effect of co- administration of NaHCO3 and/or cimetidine.
When both NaHCO3 and cimetidine were administered together, gentamicin kidney accumulation decreased ~80%, with corresponding increases in clearance and XR compared to gentamicin alone.
4. Discussion
Despite their toxicity profile, the clinical use of aminoglycosides has increased in recent years following the emergence of multidrug resistant pathogens [38] . Since the incidence of aminoglycoside nephrotoxicity is ~25%, various strategies have been proposed to circumvent this toxicity either by reducing drug accumulation in the kidney or by co-administering renoprotective compounds [1] [4] .
It is well established that aminoglycosides are substrates for megalin, a multiligand endocytotic receptor in the luminal membrane of the kidney, and this is thought to be a major pathway for accumulation of aminoglycosides on the kidney [7] [39] . Accordingly, this pathway is a proposed target to prevent aminoglycoside toxicity, and research has showed that gentamicin binding can be inhibited by megalin ligands and small peptides [10] . However, concern has been raised about the clinical consequences of interfering with megalin-mediated
![]()
Figure 2. Plot of gentamicin cumulative urinary excretion vs. time in the IPRK: Effect of co-administration of NaHCO3 and/or cimetidine.
![]()
Table 4. Gentamicin renal excretion parameters in IPRK: interaction experiments.
aGlomerular filtration rate, normalized for kidney weight; bCalculated as the ratio of cumulative urinary excretion and AUC for duration of IPRK experiment (data are corrected for kidney weight); cCalculated are ratio of clearance (Clearance/(fu × GFR)); dEstimated from mass balance analysis at end of experiment (dose-cumulative excretion-amount remaining in perfusate); eArea under the curve was estimated using trapezoidal rule; *Indicates values that are significantly different from gentamicin alone; **Indicates values that are significantly different from NaHCO3 treatment group; ***Indicates values that are significantly different from Cimetidine + NaHCO3 treatment group.
endocytosis [4] . Additionally, several preclinical studies have demonstrated reduced aminoglycoside nephrotoxicity through co-administration of antioxidants [21] -[23] , so this is an avenue for further exploration.
In the present study, gentamicin excretion was evaluated in the IPRK model, a versatile ex vivo technique that can be used to study numerous aspects of renal drug disposition. Some of the earliest evidence that luminal uptake was responsible for the renal tubular uptake of gentamicin came from IPRK experiments in filtering and non-filtering kidneys [18] . The present work extended application of the IPRK to assess dose-linearity of gentamicin excretion and to probe ways to reduce kidney accumulation.
Dose linearity experiments were carried at four doses (400, 800, 1600 and 3200 mg) targeting initial concentrations from 5 - 40 mg/ml. These concentrations encompass the clinical range of expected peak levels of gentamicin following a conventional dosing regimen (1 - 2 mg/kg every 8 hours, targeting peak serum concentrations of 5 - 10 mg/ml) or a “once daily” dosing regimen (5 - 7.5 mg/kg every 24 hours, targeting peak serum concentrations as high as 30 - 40 mg/ml) [40] [41] .
Estimates of gentamicin clearance in the IPRK have been reported by Bekersky et al. (0.25 - 0.30 ml/min, reference 25) and Collier et al. (0.32 ml/min, reference 18). These findings are based on studies performed at an initial drug concentration of 10 mg/ml. The results of the present investigation are consistent with these values. The mean gentamicin clearance (not kidney weight corrected, 10 mg/ml dosing group) was 0.29 ± 0.033 ml/min.
Approximately 50% - 60% of the administered gentamicin dose was eliminated from the perfusate over the duration of the IPRK experiment (100 minutes). Comparing the temporal profiles of urinary excretion rate with perfusate concentrations (Figure 3), there is a distributional delay in the renal excretion of gentamicin; that is, the luminal transport of gentamicin (kidney Þ urine) appears to be the rate-determining step for drug excretion. However, this observation is consistent with slow removal of drug from the proximal tubule [7] , and would therefore lead to renal accumulation with successive dosing.
Gentamicin displayed nonlinear excretion in the IPRK, with significant decreases in clearance and cumulative excretion with increasing dose (Table 2). However, kidney accumulation (% dose) did not decrease with dose. Although “once a day” dosing is thought to decrease kidney accumulation through saturation of aminoglycoside reabsorption [1] [5] [9] , the nonlinear behavior seen in the present study does not support this hypothesis. Aminoglycoside uptake into the kidney involves pathways other than absorptive endocytosis [7] , and it appears that one of these pathways is responsible for the nonlinearity in gentamicin excretion in the IPRK.
One of the goals of this investigation was to test potential strategies to decrease the kidney accumulation of gentamicin: urinary alkalization and co-administration of cimetidine. Administration of NaHCO3 (0.25 mM) to the IPRK caused a significant increase in urine pH from ~7.1 to ~8.1, and this effect was constant for the duration of the experiment. Under these conditions, gentamicin excretion was significantly increased, as reflected in differences in clearance (0.40 ml/min/g vs. 0.18 ml/min/g), excretion ratio (0.47 to 0.94) and cumulative excretion (47.7% vs. 26.2%) compared to gentamicin alone.
Gentamicin tubular uptake has been studied extensively in the last decade. Ionized gentamicin binds to the acidic phospholipids on brush-border membrane of the renal tubular cell. Thus, altering the ionized fraction of gentamicin can ameliorate its nephrotoxicity. Gentamicin is weak base with a pKa = 7.4. Thus, increasing urine pH to 8.1 decreased the ionization of gentamicin to ~17%. Since luminal reabsorption of gentamicin is an electrostatic process [42] , altering the cationic charge on the molecule adversely impacted epithelial uptake of gentamicin by reduced charged affinity for the luminal membrane. However, increased gentamicin excretion was not associated with decreased kidney accumulation (Table 4).
The effect of urinary alkalization on gentamicin kidney uptake and nephrotoxicity has previously been studied in rats. Whereas Chiu et al. observed a decrease in gentamicin accumulation in the kidney cortex when urine pH was increased [43] , Elliott reported that pre-treatment with NaHCO3 was not associated with reduced gentamicin nephrotoxicity [44] . The results from these IPRK experiments suggest that while gentamicin excretion is increased
![]()
Figure 3. Comparison of the temporal changes in gentamicin perfusate concentration and urinary excretion rate following bolus dosing (800 mg) in the IPRK.
when co-administered with NaHCO3, this strategy would likely not reduce the risk of nephrotoxicity, as kidney accumulation was not altered with this treatment.
IPRK experiments also investigated the effect of co-administration of cimetidine on gentamicin excretion and kidney accumulation. Although luminal transport has been the principle target for reducing aminoglycoside accumulation in the kidney, there is evidence to suggest that gentamicin is secreted across the basolateral membrane [45] [46] . Cimetidine is a known inhibitor of basolateral organic cation transport (OCT2). Although cimetidine had no apparent effect on gentamicin clearance and urinary excretion in the IPRK (Table 4), there was a slight increase in AUC (Figure 1) and a significant reduction in kidney accumulation (~50%). These results indicate that basolateral transport (blood ® kidney) is a promising target for reducing aminoglycoside accumulation. Whereas tubular secretion may contribute significantly to the urinary excretion of aminoglycosides (i.e., inhibition of basolateral transport would not alter renal clearance), the pathway appears to be an important determinant of aminoglycoside toxicity.
In a final set of experiments, the effect of both basolateral transport inhibition and urinary alkalization on gentamicin disposition in the IPRK was explored. Co-administration with both cimetidine and NaHCO3 not only increased renal excretion of gentamicin, but kidney accumulation was also reduced 80% (Table 4). Thus, it appears that urinary alkalization combined with basolateral transport inhibition is a potential strategy to limit the renal accumulation of gentamicin and to reduce the risk of drug-induced nephrotoxicity.
5. Conclusion
While further studies are needed to confirm the results of this investigation and to evaluate the synergistic protective effect of NaHCO3 and cimetidine, an advantage of this approach over other proposed strategies is that both compounds are clinically available. A recent clinical study found that oral administration of sodium bicarbonate (4 g every 8 hours) was able to achieve a urine pH above 8 with no apparent adverse effects, although the authors acknowledge that more research is needed to investigate efficacy of longer periods of treatment [46] . Cimetidine is a commercially available medication used to treat gastric ulcers, gastroesophegeal reflux disease, and other conditions. While further studies are needed to confirm that kidney accumulation of gentamicin can be reduced at therapeutic doses of cimetidine, the findings of the present investigation are promising and merit continued exploration.
Funding Information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary
![]()
Table S1. Summary of calibration curve of gentamicin HPLC method in IPRK perfusate and KHS buffer.
aData presented as mean ± standard deviation of six calibration curves; bData obtained after the application of standard addition method.
![]()
Table S2. Precision of gentamicin quantification method by HPLC in IPRK perfusate and KHS buffer.
aData presented as mean ± standard deviation of six replicate injections at each concentration; bData obtained after standard addition method was utilized.
*Corresponding author.
![]()
*Corresponding author.