Synthesis of Fluorinated Heterobicyclic Nitrogen Systems Containing 1,2,4-Triazine Moiety as CDK2 Inhibition Agents ()
1. Introduction
1,2,4-triazines and their condensed analogues have shown to display wide-ranging applications in medicinal and pharmaceutical chemistry. The NCNN group of 1,2,4-triazine ring is an essential part in various biological activities. Tirapazamine as antitumor [1] , Lamotrigine as anti-epileptic drug [2] , and fused 1,2,4-triazines as antimicrobial [3] -[5] , anti HIV [6] antimycobacterial [7] , antiviral [8] [9] , anxiolytic [10] and antidepressant [11] agents are already reported in literature. Heterobicyclic nitrogen systems containing 1,2,4-triazine moiety have also shown anti-HIV and anticancer activities [12] -[16] . However, introduction of fluorine atoms to these bioactive heterocycles often improves their pharmacological properties mainly due to increased membrane permeability, enhanced hydrophobic bonding and stability against metabolic transformation owing to the strength of the C-F bond [17] -[21] . Also, introduction of fluorine in these heterobicyclic systems exhibited enhanced anti-tumor activity [20] . The search for novel cancer treatment has however entered a new post-genomic era and the emphasis now is on influencing cell signaling mechanisms such as those triggered by kinases [22] where selective inhibition of the Kinome constituents and related signaling proteins has opened up a diversity of new drug targets.
Cyclin-Dependent Kinases (CDKs) are families of serine/threonine kinases that play a well-established role in the regulation of the eukaryotic cell division cycle and have also been implicated in the control of gene transcription and other processes [23] . Dysregulation of cell cycle in cancer cells has provided a rationale for the development of small molecule inhibitors of CDK1 as novel anticancer drugs. It was believed that CDK2 was the master regulator of S phase entry. Gene knockout mouse studies of cell cycle regulators revealed that CDK2 is dispensable for S phase inhibition and progression whereby CDK1 can compensate for the loss of CDK2 and the latter was found to be involved in cell cycle independent functions such as DNA damage repair [24] .
Rational approaches to the design of fluorine-containing potential inhibitors associated with the strategic placement of fluorine in these molecules led us to design CDK2 inhibitors as possible anti-cancer agents. The aim of the present work is to synthesize and develop fluorinated fused and/or isolated heterobicyclic nitrogen systems, derived from the interaction between 3-amino,6-(4′-fluorophenyl)-1,2,4-triazines (1) and α, β-bifunc- tional oxygen, sulfur, halogen and nitrogen compounds. In this work, we evaluate the protein kinase inhibiting and cytotoxic activity of fluorinated heterobicyclic systems containing 1,2,4-triazine moiety.
2. Experimental
2.1. Chemicals and Methods
Melting points were determined using an electrothermal Bibby Stuart Scientific melting point apparatus and are uncorrected. The infrared (IR) spectra were recorded on a Perkin-Elmer FT-IR infrared spectrophotometer using the KBr pellet technique. Electronic absorption spectra were recorded in DMF using a Shimadzu UV-Visible 1650 PC spectrophotometer. 1H and 13C NMR spectra were recorded on a Bruker DPX-400 FT NMR spectrometer using tetramethylsilane ( internal standard DMSO-d6) as a solvent (Chemical shifts in δ, ppm). 19F NMR Spectra were determined at 84.25 MHz using hexafluorobenzene as a solvent. Splitting patterns were designated as follows: s: singlet; m: multiplet. Mass spectra were measured on a GCMS-Q 1000 Ex spectrometer. Elemental analyses were performed on a 2400 Perkin Elmer Series 2 analyzer and the found values were within ±0.4% of the theoretical values. Follow up of the reactions and checking the homogeneity of the compounds were made by TLC on silica gel-protected aluminum sheets (Type 60 F254, Merck) and the spots were detected by exposure to UV-lamp.
2.1.1. 3-Amino-5,6-di(4׳-fluorophenyl)-1,2,4-triazine (1)
A mixture of 4,4׳-bifluorobenzil (0.01 mol) and aminoguanidine bicarbonate (0.01 mol) in n-butanol [28] was refluxed for 4 h. The solid obtained after cooling the mixture was filtered and crystallized from ethanol to give orange crystals, yield 75%, m.p. 208˚C - 209˚C. IR (n/cm−1): 3200 (NH2), 1620 (deformation, NH2), 1600 (C=N), 1250 (C-F) and 880, 820 (p-substituted phenyl). 1H-NMR (DMSO d6) δ: 2.89 - 2.51 (br. s, 2H, NH2), 7.31 - 7.39, 7.39 - 7.44 (each m, 8H, aromatic protons), 7.0 - 6.61 (m). 13C-NMR (DMSO d6) δ: 164,156, 148, 136 - 131, 129.8 - 127.7. Anal. Calcd: C, 63.15; H, 3.40; N, 21.67; F, 11.76% for C17H5N5F2 (323). Found: C, 62.98; H, 3.25; N, 21.50; and F, 11.6%.
2.1.2. 3-Amino-5,7-di(4'-fluorophenyl)imidazo[3,2-b][1,2,4-]triazine (2)
A mixture of 1 (0.01 mol) and chloroacetonitrile (0.01 mol) in DMF was refluxed for 4 h. The solid obtained on cooling the reaction mixture was filtered and crystallized from dioxan to give 2 as yellowish crystals, yield 70%, m.p. 141˚C - 142˚C. IR (n/cm−1): 3350, 3228 (NH2, NH), 31.86 (aromatic CH), 1623 (deformation NH2), 1601, 1517 (C=N), 1350 (NCN), 1226 (C-F). 1H-NMR (DMSO d6) δ: 7.89, 7.4, 7.37, 7.28, 7.24, 7.166, 7.00 and 6.94 (m, 8H, aromatic protons), 6.89, 6.717 (d, d of H adjacent of F), 4.01 - 3.97 (s, 2H, NH2). 13C-NMR (DMSO d6) δ: 164, 155, 147, 132, 131 - 130.4, 115, 114, 77.88 - 77.46, 59.68. M/S (Int. %): 323 (8.89), 282 (33.01), 214 (100). Anal. Calcd: C, 62.96; H, 3.08; N, 17.28; F, 11.72%; for C17H10N4F2O (324). Found: C, 62.79; H, 2.88; N, 17.15; and F, 11.55%.
2.1.3. 6,7-Di(4'-fluorophenyl)-2,3-dihydro-3-oxo-imidazo[3,2-b][1,2,4-]triazine(3)
A mixture of 1 (0.01 mol) and monochloroacetic acid (0.01 mol) in DMF was heated for 15 min. and then left to cool at room temperature. The solid obtained was filtered and crystallized from dioxan to give 3 as faint yellowish crystals, yield 65%, m.p. 193˚C - 194˚C. IR (n/cm−1): 3090 (ArCH), 1640 (C=O ↔ C-OH), 1480 (deformation CH2), 2950 (aliphatic CH), 1350 (NCN), and 1227 (C-F). 1H-NMR (DMSO d6) δ: 7.61, 7.41, 7.39, 7.38, 7.32, 7.31, 7.306 and 7.301 (m, 8H, aromatic protons), 7.29 (s, 1H, CH=), 6.98 - 6.97, 6.95 and 6.54 (d, d of H adjacent of F), 2.52 - 2.51 (s, 2H, CH2). 13C-NMR (DMSO d6) δ: 164, 155, 147, 132.1, 131 - 130.4, 115, 114 and 77.77 - 77.34. Anal. Calcd: C, 62.96; H, 3.08; N, 17.28; F, 11.72%; for C17H10N4F2O (324). Found: C, 62.76; H, 2.91; N, 17.01; and F, 11.55%.
2.1.4. 7,8-Di(4'-fluorophenyl)-2,3-dihydro-pyrimido[3,2-b][1,2,4-]triazin-2,4-dione (4)
A mixture of 1 (0.01 mol) and diethylmalonate (0.01 mol) in THF was refluxed for 4 h. On cooling the reaction mixture, a yellowish solid separated which was crystallized from THF to give 4 as yellow crystals, yield 72%, m.p. 199˚C - 200˚C. UV (EtOH): λmax 346 nm. IR (n/cm−1): 3091 (Ar-CH), 2980 (aliphatic CH), 1680, 1650 (2C=O), 1576 (C=N) 1492 (deformation CH2), 1381 (NCN), 1225 (C-F), 919, 867 and 815 (p-substituted phenyl). 1H-NMR (DMSO d6) δ: 7.69 - 7.29 (m, 8H, aromatic protons), 6.98 - 6.67 (d, d, s, H adjacent F), 3.65 - 3.613 (t, 2H, CH2). 13C-NMR (DMSO d6) δ: 164.01, 162.93, 155, 147.86, 132.18, 131.83 - 131.29, 130.54, 130.49, 115.03, 114.88, 77.97 - 77.54, 67.22 and 40.03-39.33. Anal. Calcd: C, 61.36; H, 2.84; N, 15.90; and F, 10.79%; for C18H10N4F2O2 (324). Found: C, 61.86; H, 2.56; N, 15.78; and F, 10.58%.
2.1.5. 4-Amino-2-oxo-7,8-di(4'-fluorophenyl)pyrimido[3,2-b][1,2,4-]triazine (6)
A mixture of 1 (0.01 mol) and ethyl cyanoacetate (0.01 mol) in THF was refluxed for 4 h. The solid obtained after cooling the reaction mixture was filtered and crystallized from ethanol to give deep-yellow crystals, yield 65%, m.p. 198˚C - 200˚C. UV (EtOH): λmax 346 nm. IR (n/cm−1): 3300 (NH2), 1670, 1610 (deformation NH2), 1229 (C-F), 828 and 810 (p-substituted ring). 1H-NMR (DMSO d6) δ: 7.40 - 7.29 (m, 8H, aromatic protons), 6.971 (s, 1H, =CH cyclic), 6.96 - 6.69 (d, d, s, H adjacent of F), 3.62 (s, 2H, NH2). 13C-NMR (DMSO d6) δ: 164.01, 154.9, 147.8, 132.16, 131.81 - 130.48, 115, 114.8, 77.78 - 77.57 and 67.21. M/S (Int. %): 351 (8.18), 307 (28.13), 214 (100). Anal. Calcd: C, 61.53; H, 3.13; N, 19.94; and F, 10.82%; for C18H11N5F2O (351). Found: C, 61.33; H, 3.02; N, 19.58; and F, 10.69%.
2.1.6. 7,8-Di(4'-fluorophenyl)-3-(arylidene)pyrimido[3,2-b][1,2,4-]triazin-2,4-dione (5)
A mixture of 4 (0.01 mol) and p-chlorobenzaldehyde (0.01mol) in EtOH was refluxed for 1h and then allowed to cool at room temperature. The solid thus obtained was filtered and crystallized from ethanol to give 5 as yellowish crystals, yield 78%, m.p. 208˚C - 209˚C. IR (n/cm−1): 3024 (Ar-CH), 1680, 1660 (2C=O), 1610 (C=C), 1576 (C=N), 1382 (NCN), 1227 (C-F), 820 and 815 (p-substituted ring). 1H-NMR (DMSO d6) δ: 9.9 (s, 1H, CH=), 7.76 - 7.75, 7.70 - 7.60 (m, 8H, aromatic protons), 7.35 - 7.20 (m, 4H, aryl protons), and 6.95 - 6.37 (d, d, s, H of adjacent F). Anal. Calcd: C, 63.15; H, 2.73; N, 11.78; F, 8.0; and Cl, 7.57%; for C25H13N4F2ClO2 (475). Found: C, 62.88; H, 2.60; N, 11.58; F, 7.69; and Cl, 7.49%.
2.1.7. 7,8-Di(4'-fluorophenyl)-4-arylidino-pyrimido[3,2-b][1,2,4-]triazin-2-one(7)
A mixture of 6 (0.01 mol) and p-chlorobenzaldehyde (0.01 mol) in EtOH was refluxed for 1h and subsequently left to cool at room temperature. A solid appeared which was filtered and crystallized from ethanol to give yellow crystals, yield 82%, m.p. 212˚C - 214˚C. IR (n/cm−1): 3092 (Ar-CH), 1668 (C=O), 1602 (C=N), 1226 (C-F), 919, 858 and 815 (p-substituted ring). 1H-NMR (DMSO d6) δ: 7.85 - 7.81 (m, 4H, aromatic H), 7.40 - 7.28 (m, 8H, aromatic H), 6.99 - 6.97 (d, d, s, H of adjacent F), 6.838 (1H, CH=N). 13C-NMR (DMSO d6) δ: 163.96, 161.24, 154.92, 147.68, 132.32, 131.95, 130.58 - 130.52, 115.03, 115.02, 114.89, 114.88, 78.25 - 77.81 and 40.16. Anal. Calcd: C, 63.29; H, 2.95; N, 14.76; F, 8.01; and Cl, 7.59%; for C25H14N5F2ClO (474). Found: C, 62.98; H, 2.75; N, 14.49; F, 7.78; and Cl, 7.38%.
2.1.8. Ethyl 3-(carboxyamino)-5,6-di(4׳-fluorophenyl)-1,2,4-triazine (8)
A mixture of 1 (0.01 mol) and ethyl chloromethanoate (0.01 mol) in benzene-TEA mixture was refluxed for an hour. The solid obtained after cooling the reaction mixture was filtered and crystallized from THF to give 8 as faint yellow crystals, yield 60%, m.p. 178˚C - 180˚C. IR (n/cm−1): 3300 (NH), 3080 (Ar-CH), 2980 (R-CH), 1676 (C=O), 1483 (deformation CH3), 1381 (NCN), 1226 (C-F), 1010 (O-C-O), 840, and 815 (p-substituted ring). 1H-NMR (DMSO d6) δ: 8.87 (s, 1H, NH), 7.68 - 7.40, 7.39 - 7.29 (each m, 8H, aromatic H), 7.03 - 7.00, 6.94 - 6.82 (d, d, s, H adjacent F), 4.20 (t, 2H, CH2O), and 1.4 - 1.2 (s, 3H, CH3). 13C-NMR (DMSO d6) δ: 164.87, 161.81, 160.98, 157.05, 147.64, 146.91, 141.26, 132.14, 132.08, 130.86 - 127.10, 115.40, 115.25, 114.96, 114.45, 78.46 - 78.03, 39.98, and 14.19. Anal. Calcd: C, 60.67; H, 3.93; N, 15.73 and F, 10.67%; for C18H14N4F2O2 (356). Found: C, 60.41; H, 3.64; N, 15.61 and F, 10.55%.
2.1.9. N4-(5’-6’-diaryl-1,2,4-triazin-3’-yl)semicarbazide (10)
A mixture of 8 (0.01 mol) and hydrazine hydrate (0.01 mol) in EtOH was refluxed for 2 h, and then cooled at room temperature. The solid obtained was filtered and crystallized from ethanol to give 10 as faint yellow crystals, yield 60%, m.p. 198˚C - 200˚C.
IR (n, cm−1): 3334, 3269 (NH, NH2), 3092(Ar-CH), 1660 (C=O), 1228 (C-F), 860 and 810 (p-substituted ring). 1H-NMR (DMSo-d6): 8.8 (s, 1H, NH), 7.72-7.25 (m, 8H aromatic H), 6.98 - 6.67 (m, d, d, s, H, adjacent F) and 3.16 (s, 2H, NH2). 13C-NMR(DMSO-d6): 164.02, 161.29, 155.02, 147.86, 132.17, 131,82, 131.35, 131.29, 130.54 - 130.49, 115.13, 115.02, 114.88, 77.94 - 77.53 and 40.14 - 39.19. Analy. Calcd.: C, 56.14; H, 3.50; N, 24.26 and F, 11.11%; for C16H12N6F2O (342). Found: C, 55.89; H, 3.35; N, 24.39; and F, 11.01%.
2.1.10. N4-(5׳-6׳-Di(4׳-fluorophenyl)-1,2,4-triazin-3׳-yl)thiosemicarbazide (11)
A mixture of 1 (0.01 mol) and carbon disulfide (0.01 mol) in 1% KOH solution was stirred for 2 h at room temperature to give the intermediate 9. Now a mixture of 9 (0.01mol) and hydrazine hydrate (0.01mol) in EtOH was refluxed for 2 h and subsequently cooled in an ice-bath for few minutes to form a brownish yellow solid. The solid was filtered and crystallized from ethanol to give 11 as orange crystals, yield 78%, m.p. 203˚C - 204˚C. IR (n/cm−1): 3314, 3229, 3192 (NH, NH, NH). 1381 (NCN), 1225 (C-F), 1157 (C=S) and 850 (p-substituted ring). 1H-NMR (DMSO-d6): 772 - 7.29 (m, 8H, aromatic H), 6.98 - 6.72 (m, d, d, s, H, adjacent F) and 3.14 (s, 2H, NH2). 13 C-NMR (DMSO-d6): 164.0, 162.2, 161.28, 154.98, 147.81, 132.21, 131.85 - 130.50, 115.03, 114.88, 78.04 - 77.61 and 40.02-39.19. Analy. Calcd: C, 53.63; H, 3.35; N, 23.46; and F, 10.61; S, 8.93%, for C16H12N6F2S (358), Found: C, 53.38; H, 3.20; N, 23.41; and F, 10.38; S, 8.78%.
2.1.11. 5,6-Di(4׳-fluorophenyl)-3-(2׳,3׳-dihydro-3׳-oxo-1׳,2׳,4׳-triazol-4׳-yl)-1,2,4-triazine (12)
A mixture of 10 (0.5 g) and triethyl orthoformate (10 ml) was refluxed for 2h and then cooled at room temperature. The produced solid was filtered and crystallized from dioxan to give 12 as faint yellowish crystals, yield 65%, m.p. 190˚C - 192˚C. IR (n/cm−1): 3192 (NH), 1679 (C=O), 1572(C=N), 1382 (NCN),1227 (C-F), 880 and 816 (p-substituted ring). 1H-NMR (DMSO-d6): 8.81 (s, 1H, NH), 7.62 - 7.27 (m, 8H, aromatic H), 6.966 - 6.92 (d, d, s, H, adjacent F) and 6.60 (s, 1H,CH=N):13CNMR (DMSO-d6): 164.02, 161.40, 154.9, 132.16, 131.28, 130.53 - 130.48, 115.02, 114.88 and 77.93 - 77.49. Analy. Calcd: C, 57.95; H, 2.84; N, 23.86; and F.10.79%, for C17H10F2O (352). Found: C, 57.55; H, 2.70; N, 23.59; and F, 10.61%.
2.1.12. 5,6-Di(4׳-fluorophenyl)-3-(3׳-mercapto-5׳-hydroxy-1׳,2׳,4׳-triazol-4׳-yl)-1,2,4-triazine (13)
A mixture of 11 (0.01 mol) and carbon disulfide (0.01 mol) in dimethyl formamide was refluxed for 2 h. The reaction mixture was cooled in ice-bath to form a solid which was filtered and crystallized from ethanol to give 13 as orange crystals, yield 65%, m.p. 210˚C - 212˚C. IR (n/cm−1): 3259, 3192 (NH, NH), 1658 (C=O), 1389 (NCN), 1225 (C-F), 1156 (C=S), 880 and 829 (p-substituted ring). 1H-NMR (DMSO-d6): 13.3, 12.8 (each s, NH, NH), 7.91 - 7.28 (m, 8H, aromatic H) and 6.98 - 6.69 (m, d, d, s, H adjacent F). 13CNMR (DMSO-d6):195 (164.0), 161.28, 154.99, 147.83, 132.20 131.84, 131.29 - 130.49, 115.03, 114.88 and 78.01 - 77.58. Analy. Calcd: C, 53.12; H, 2.60; N, 21.87: F. 9.89; and S, 8.33%, for C17H10N2SO (384), Found: C, 52.89; H, 2.39; N, 21.55; F, 9.69; and S, 8.01%.
2.1.13. 5,6-Di(4׳-fluorophenyl)-3-(6׳-methyl-3׳-hydroxy-5׳-oxo-1׳,2׳,4׳-triazin-4׳-yl)-1,2,4-triazine (14)
Equimolar mixture of 10 and sodium pyruvate in 5% NaOH solution (50 ml) was refluxed for 2 h, cooled then poured onto ice-HCl. The solid obtained was filtered and crystallized from ethanol to give 14 as faint yellow crystals, yield 65%, m.p. 213˚C - 214˚C. IR (n/cm−1): 3372, 3297 (OH+NH), 1700, 1626 (CO, CONH), 1567 (C=N), 1483 (deformation CH3) 1379 (NCN), 1224 (C-F), 1155 (C=S), 829 and 810 (p-substituted ring). 1H-NMR (DMSO-d6): 13.481 (s, 1H, NH), 7.65, 7.41, 7.39, 7.22, 7.20, 7.19, 6.985, 6.978 (8H, aromatic H), 6.971 - 6.964, 6.957 - 6.950 (m, d, d, s, H of F) and 1.903 (s, 3H, CH3).13C-NMR (DMSO-d6): 195.63, 164.73, 163.06, 161.66, 153.43, 140.91, 131.69, 131.11, 131.09 - 129.99, 115.21, 115.06, 114.95, 77.88 - 77.44, 40.02, and 39.19. Analy. Calcd: C, 57.86; H, 3.04; N, 12.13; and F, 9.64%, for C19H12N6 F2O2 (394). Found: C, 57.49; H, 2.89; N, 21.01; and F, 9.33%.
2.1.14. 5,6-Di(4׳-fluorophenyl)-3-(3׳-mercapto-1,2,4-triazol-4׳-yl)-1,2,4-triazine (15)
A mixture of 11 (0.01 mol) and triethyl orthoformate (0.01 mol) was refluxed for 2 h and then cooled at room temperature. The solid obtained was filtered and crystallized from dioxan to give 15 as yellow crystals, yield 72%, m.p. 183˚C - 184˚C. IR (n/cm-1): 3298, 3191 (NH), 1610, 1576 (C=N), 1381 (NCN), 1225 (C-F), 1157 (C=S), 840 and 810 (p-substituted ring). 1H-NMR (DMSO-d6): 10.03 (S, 1H, NH=S), 7.60 - 511, 7.48 - 7.27 and 7.0 - 6.55 (m, 9H, aromatic H). Analy. Calcd: C, 55.43; H, 2.71; N, 22.82; F.10.32 and S, 8.69%, for C17H10N6F2S (368). Found: C, 55.21; H, 2.33; N, 22.55; F.10.01 and S, 8.49%.
2.1.15. 5,6-Di(4׳-fluorophenyl)-3-(3׳,5׳-dimercapto-1׳,2׳,4׳-triazol-4׳-yl)-1,2,4-triazine (16)
A mixture of 11 (0.01 mol) and carbon disulfide (0.01 mol) in DMF was refluxed for 2 h and then cooled at room temperature. The solid obtained was filtered and crystallized from methanol to give 16 as orange crystals, yield 80%, m.p. 203˚C - 204˚C. IR (n/cm−1): 3197 (NH), 3150 (NH), 1595 (C=N), 1564 (C=N), 1382 (NCN), 1224 (C-F), 1151(C=S), 831 and 810 (p-substituted ring). 13C-NMR (DMSO-d6): 163.99162.90، ، ،161.47 161.26 ، ،154.96 147 ، 132.25 ، 132.23 ، 131.89 ، 131.87 131.31 130.56 ، ، 130.50 ، 115.02 ، 114.88 ، 77 and 77.69-78.12. Analy. Calcd: C, 51.0; H, 2.50; N, 21.0; F, 9.5; and S, 16.0%, for C17H10N6F2S2 (400). Found: C, 50.85; H, 2.31; N, 20.88; F, 9.33; and S.15.79%.
2.1.16. 5,6-Diaryl-3-(6׳-methyl-3׳-mercapto-6׳-oxo-1׳,2׳,4׳-triazin-4׳-yl)-1,2,4-triazine (17)
A mixture of 11 (0.01 mol) and sodium pyruvate (0.01 mol) in 5% NaOH aq. (20 ml) was refluxed for 3h. The reaction mixture was cooled and then poured on ice cold HCl solution. The solid obtained was filtered and crystallized from ethanol to give 17 as yellow crystals, yield 60%, m.p. 220-222˚C. IR (n/cm−1): 3197 (NH), 2906, 2855(CH3), 1666 (C=C), 1586 (C=N), 1472 (deformation CH3), 1367 (NCN), 1224 (C-F), 1158 (C=S), 872 and 792 (p-substituted ring). 1H-NMR (DMSO-d6): 11.86 (S, 1H, NH), 7.75 - 7.38, 7.30 - 7.28 (each, m, 8H, aromatic H), 6.97 - 6.69 (d, d, s, H-CF), and 1.08 (S, 3H, CH3). 13C-NMR (DMSO-d6): 180, 163.98, 162.91, 161.27, 154.96, 147.84, 132.21, 131.85, 131.36, 131.30, 130.55 - 130.02, 115.02, 114.88, 78.03 - 77.60 and 39.20. Analy. Calcd: C, 55.60; H, 2.92; N, 20.48; F, 9.26; and S.7.80%, for C19H12N6F2SO (410). Found: C, 55.39; H, 2.80; N, 20.29; F.9.01 and S, 7.55%.
2.1.17. N,N׳-[5,6-di(4¢fluorophenyl)-1,2,4-triazin-3¢-yl]thiourea (18)
Equimolar amount of compounds 1 and 9 in ethanol (50 ml) was refluxed for 2 h. The reaction mixture was cooled in an ice bath to form a solid which was filtered and crystallized from ethanol to give 18 as faint yellow crystals, yield 65%, m.p. 193˚C - 194˚C. IR (n, cm−1): 3300, 3289, 3192 (C-F), 1157(C=S), 827 and 814 (p- substituted ring). Analy. Calcd: C, 60.98; H, 2.95; N, 18.36; F, 12.45; and S.5.24%, for C31H18N8F4S (610). Found: C, 60.65; N, 2.80; F, 12.13; and S, 5.00%.
2.1.18. N,N׳-Di[di(4׳-fluorophenyl)1,2,4-triazin-3-yl]-thiobarbituric Acid (19)
A mixture of 18 (0.01 mol) and malonic acid (0.01 mol) in glacial acetic acid (50 ml) was refluxed for 4h. The reaction mixture was cooled and then poured onto ice. The solid obtained was filtered and crystallized from ethanol to give 19 as yellow crystals, yield 60%, m.p. 300˚C - 302˚C. UV (Ethanol): λmax 334 nm. IR (n/cm−1): 2980, 2868 (aliphatic CH), 1663 (C=O), 1595, 1561(C=N), 1366 (NCN), 1223 (C-F), 1156 (C=S), 854 and 820 (p-substituted ring). 1H-NMR (DMSO-d6): 13.52 (s. IH, OH), 7.82 - 7.00 (m, 16H, aromatic H), 6.98, 6.97, 6.85(d, d, S, H, of C-F) and 2.499 (S, CH2). 13C-NMR (DMSO-d6):180, 165.68, 164.65, 163.27, 162.98, 161.62, 154.93, 140.95, 131.95 - 130.14, 115.22, 114.89, 78.28 - 77.85 and 24.88. Analy. Calcd: C, 60.15; H, 2.66; N, 16.49; F, 11.10; and S, 4.55%. for C34H18N8F4SO2 (678). Found: C, 59.89; H, 2.51; N, 16.30; F, 10.89; and S, 4.40%.
2.1.19. N,N׳-Di[di(4׳-fluorophenyl)1,2,4-triazin-3-yl]-5-arylidene-thiobarbituric Acid (20)
Equimolar quantity of 19 and 4-chlorobenzaldehyde in ethanol (50 ml), piperidine (0.5 ml) was refluxed for 6h. The reaction mixture was cooled and neutralized with few drops of acetic acid. The solid precipitated was filtered and crystallized from ethanol to give 20 as yellow crystals, yield 60%, m.p. 310˚C - 312˚C. UV (EtOH): λmax 346 nm. IR (n, cm−1): 3080 (aromatic CH), 2920, (aliphatic CH), 1710, 1680(2C=O), 1385 (NCSN), 1250 (C-F), 1190 (C-S), 880 and 810 (p-substituted ring). 1H-NMR (DMSO-d6): 7.66 - 7.64, 7.38 - 7.36, 7.29 - 7.27, (each, m, 21H, aromatic H), 6.61, 6.96 and 6.92, (CH=, d, d, s, of H adjacent to the F atoms). 13C-NMR (DMSO-d6): 180, 164, 162.94, 162.36, 161.30, 155.02, 147.89, 132.14, 131.79, 131.34, 131.28, 130.53, 130.48, 115.03, 114.88, 77.90 - 77.47, 66.42 and 40.12 - 39.17. Analy. Calcd: C, 61.42; H, 2.62; N, 13.48; F, 9.48, Cl, 4.49; and S, 3.99%, for C41H21N8FClSO2 (801). Found: C, 61.01; H, 2.55; N, 12.98; F, 9.03; Cl. 3.99 and S, 3.55%.
3. Results and Discussion
3.1. Chemistry
3-Amino-1,2,4-triazines are important intermediates in the synthesis of heterobicylic systems. Also, 5,6-di- phenyl-1,2,4-triazine containing functional groups at position-3, especially amino and/or hydrazine groups, are used as nucleophilic reagents (electron donors) towards π-acceptors carbonitriles [25] . In addition, in most of these reactions, DMF is used as a strong polar aprotic solvent which accelerates the SN2 reactions [26] .
Nucleophilic attack on exo C=N bonds of 1,2,4-trizaine provides a direct and convenient method for functionalization of 1,2,4-triazines, which makes it possible to introduce various substituents in a single reaction. Thus, it is quite probable that their reactions with bifunctional nucleophiles would result in polycyclic compounds via attack by the second nucleophilic center at another carbon atom of hetero-ring [26] .
The presence of hetero atoms results in significant changes in the cyclic molecular structure, due to the availability of unshared pairs of electrons on nitrogen atoms and the difference in electronegativity between heteroatoms and carbons within the closed system.
The starting material 3-amino5,6-di(4׳-fluorophenyl)-1,2,4-triazine (1) was prepared from refluxing amino-guanidine bicarbonate with 4,4׳-difluorobenzil in n-ButOH [27] (Scheme 1). Structure of compound 1 was deduced from elemental analysis and spectral data. IR spectrum showed an absorption bands at 3200, 1620 cm−1 for NH2 group, and 1250 cm−1 for C-F. 1H-NMR (DMSO-d6) recorded δ at 2.89-1 (br, s, 2H, NH2), 7.31 - 7.34, 7.39 - 7.44 ppm for 8 aromatic protons with δ at 7.0-6.61 ppm as a characteristic doublet and a signal for the adjacent H to the F atom. 13C-NMR spectrum exhibited a resonance signals for carbons at δ 127.7 - 129.8 for aromatic carbons and four other peaks at δ 136, 148, 156, and 161 ppm for C=N, C-N, C-F and C-NH2 respectively.
X-ray analysis of 1,2,4-triazine ring reveal that the ring is slightly distorted due to the asymmetry induced by the electronegativity of nitrogen atoms and two intermolecular hydrogen bonds that stabilized the 1,2,4-triazine structure [28] .
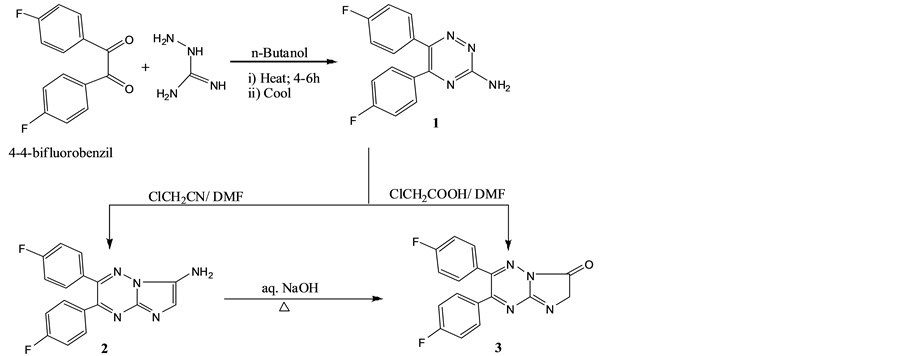
Scheme 1. Synthesis of 2 and 3.
The ring closure of 3-amino-5,6-di(4׳-fluorophenyl)-1,2,4-triazine (1) with chloroacetonitrile in boiling DMF produced 3-amino-6,7-di(4׳-fluorophenyl)imidazolo[3,2-b][1,2,4]triazine (2), while reaction of 1 with monochloro acetic acid in refluxing DMF yielded 2,3-dihydro-6,7-diaryl-imidazolo[3,2-b][1,2,4]triazin-3-one (3). Moreover, compound 3 was also obtained via basic hydrolysis of 2 by warming in aqueous NaOH.
Structure of compound 2 showed a characteristic bands at 3350 and 1623 cm−1 attributed to NH2 group while that of compound 4 had a characteristic band at 1660 cm−1 for C=O, in addition to bands at 2950 and 1480 cm−1 for CH2 group and an additional absorption at 1226 cm−1 for C-F. Mass spectra of 3 showed a molecular ion peak at m/z 323, with a base peak at m/z 214 for 4,4׳-difluorophenyl acetylene radical [29] .
Ring closure reactions of compound 1 with diethyl malonate and/or ethyl cyanoacetate in refluxing THF afforded 1,2,3,4-tetrahydro-7,8-di(4′-fluorophenyl)-pyrimido[3,2-b][1,2,4]traizin-2,4-dione (4) and 4-amino-7, 8-di(4′-fluorophenyl)-pyrimido[3,2-b][1,2,4]traizin-2-one (6), respectively. The condensation of both 4 and 6 with aromatic aldehyde in boiling ethanol?piperidine gave the arylidene 5 and the Schiff base 7 (Scheme 2).
Structures of both compounds 4 and 6 were elucidated from their analytical and spectral data. IR spectrum of 4 showed absorption bands at 1680 and 1650 cm−1 for the two C=O groups, while that of 6 recorded absorption bands at 3300, 1670 cm−1 due to NH2 and C=O groups, respectively. Mass spectrum of 6 recorded a molecular ion peak at 351 m/z and a base peak at 214 m/z. UV absorption spectrum of 5 showed a higher maximum (λmax = 351 nm) than that of 4 (λmax = 346 nm), due to the extended conjugation in the heterocyclic ring in the former. 1H-NMR of 5 showed a prominent peak at δ 8.2 ppm due to C=CHAr. 13C-NMR of compound 7 showed the resonance signal of exo N=CH in addition to an endo N=C of 1,2,4-triazine.
The treatment of compound 1 with ethyl chloroformate in benzene and triethylamine and/or CS2/KOH led to the formation of ethyl carboxylate 8 and the intermediate 9, respectively. Hydrazinolysis of 8 and 9 by refluxing with hydrazine hydrate in ethanol afforded N4-substituted semicarbazide/thiosemicarbazide 10 and 11, respectively (Scheme 3). IR spectra of both compounds 10 and 11 showed C=O and C=S at 3400, 3100 cm−1 for NH, NH2 groups, respectively.
Ring closure reactions of compound 10 with triethylorthoformate, carbondisulfide, dimethylformamide and/or sodium pyruvate (NaOH solution) led to the direct formation of 3-(2′,3′- dihydro-3′-oxo-1,2,4-triazol-4′- yl)-5,6- di(4′-fluorophenyl)-1,2,4-triazine(12), 3-(3′-oxo-1,2,3,4-tetrahydro-5′-thioxo-1,2,4-triazol-4′-yl)-5,6-di-(4′-flu- orophenyl)-1,2,4-triazine (13) and 3-(3′,4′-dihydro-3′,5′-dioxo-6′-methyl-1′,2′,4′-triazin-3′-yl)-5,6-di (4′-fluoro- phenyl)-1,2,4-triazine (14) (Scheme 4).
IR spectra of 12 and 14 evealed absorption bands at 3150 - 3100 and 1680 - 1660 cm−1 for NH and C=O groups, respectively. 1H-NMR spectrum of 14 showed resonance signals at δ 1.95, 13.48 and 8-6.6 ppm for CH3, NH and aromatic protons respectively. However compound 13 recorded a resonance C=S at δ 195 ppm. Mass spectra of 13 exhibit a molecular ion peak at m/z 384 and a base peak at m/z 214 due to 4,4'-difluorophenyl acetylene radical [29] .
Under the same experimental conditions, ring closure reactions of compound 11 with triethylorthoformate, carbondisulfide and sodium pyruvate resulted in 3-(2′,3′- dihydro-3′-thioxo-1′,2′,4′-triazol-4′-yl)-5,6-di(4′-fluo-
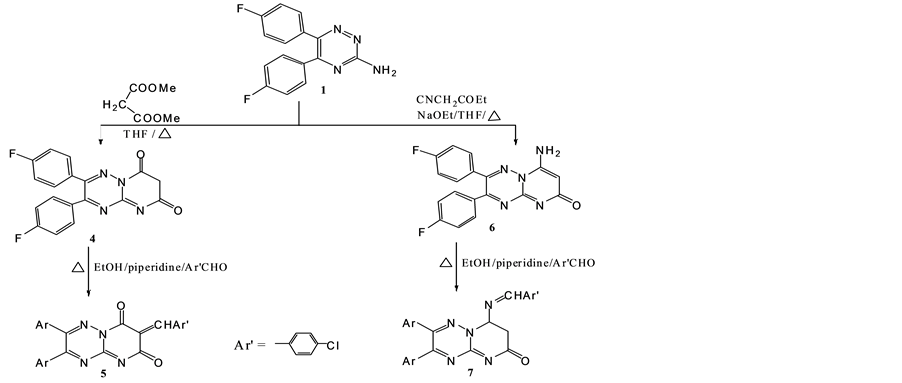
Scheme 2. Synthesis of 5 and 7.
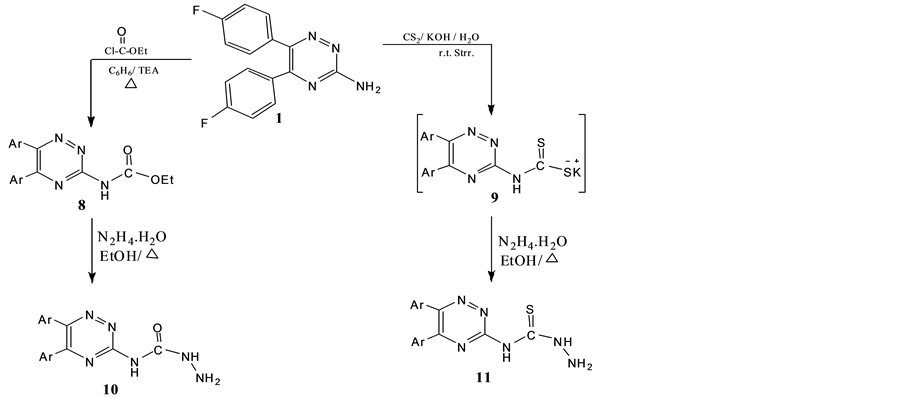
Scheme 3. Synthesis of 10 and 11.
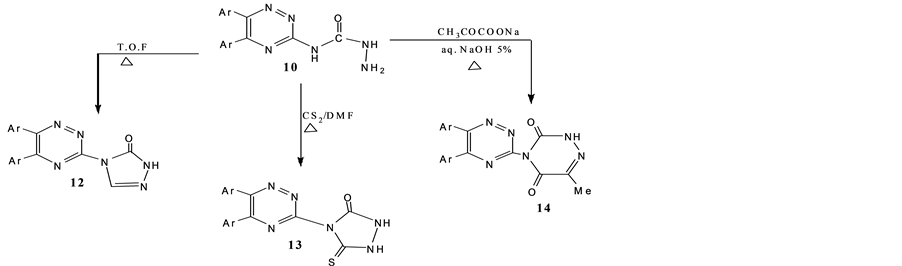
Scheme 4. Synthesis of 12-14.
rophenyl)-1,2,4-triazine (15), 3-(1′,2′,3′,4′-tetrahydro-3′,5′-thioxo-1′,2′,4′-triazol-4′-yl)-5,6-di(4′-fluorophenyl)- 1,2,4-triazine (16) and 3-(3′,4′-dihydro-3′-thioxo-6-methyl-5′-oxo-1,2,4-triazol-4′-yl)-5,6-di(4′-fluorophenyl)- 1,2,4-triazine (17), respectively (Scheme 5). IR spectra of compounds 15-17 showed an absorption bands at 3200-3100 and 1180-1150 cm−1 for NH and C=S groups, respectively. 1H-NMR of 17 recorded resonance signals at 1.08 and 11.85 ppm for CH3 and NH protons, respectively. Also, 13C-NMR exhibited resonances for C=S, C=O, C=N and CH3 at δ 180, 163 and 130 ppm respectively.
Fluorinated N,N-disubstituted-thiobarbituric acid 19 was prepared from refluxing compound 9 with 1 in ethanol to give N,N-disubstituted-thiourea 18. Ring closure reaction of 18 with malonic acid on warming with glacial acetic acid yielded compound 19 (Scheme 6). IR spectra of compound 18 showed absorption bands at 3300 - 3190 cm−1 for NH, NH of thiourea derivative which was not found in compounds 19 and 20. 1H-NMR spectrum of compound 19 recorded a resonance signal at δ 2.44 ppm for CH2 protons. Also, 13C-NMR showed a signal at δ 24.88 ppm for aliphatic carbons. IR spectrum of 20 showed absorption bands at 1680, 1660 and 1611 cm−1 for the two C=O and CH=C groups, in addition to two bands at 1255 and 1185 cm−1 for C-F and C=S groups, respectively. 13C-NMR of 20 exhibited resonance signals at δ 188, 166 and 39.48 ppm attributed to C=S, C=O and CH=C carbons, respectively. It is interesting to note that UV absorption of compound 20 recorded λmax at 334 nm which is higher than the value for compound 19 (λmax = 332), confirming ta presence of an α, β-unsaturated cyclic ketone system in the former.
3.2. Biological Evaluation
The CDK2 inhibitory activity of the synthesized compounds revealed that eleven out of the tested twenty compounds displayed variable inhibitory effects. However compounds 11, 13, 16 and 17 showed profound activity.

Scheme 5. Synthesis of 15 and 17.

Scheme 6. Synthesis of 18-20.
Compound 16 was found to be equipotent to the positive reference Olomoucine.
Further examination of the structures of the active compounds revealed that all of them have almost similar N-(amino(hydrazinyl)methyl)hydrazinecarbothioamide scaffolding as displayed in Figure 1 by bold bonds. Though these structural features are present in other active compounds but other molecular framework variants rends them less active and less sensitive towards the CDK2 enzyme. Also, the presence of mercapto-groups in fluorinated heterobicyclic nitrogen systems (11, 13, 16, 17) exhibited good effects toward cell damage (Table 1).
However, in vitro anti-tumor testing of the highly active compounds (11, 13, 16, 17) were evaluated according to the described method, under different concentrations, a sulforhodamine B (SRB) protein assay was used to estimate cell viability or growth by determining GI50, TGI and LC50 (Table 2).
The results obtained, indicated that all the tested compounds were effective towards some types of tumors. Compound 11 was active against non-small cell lung cancer, renal cancer and breast cancer cell lines, while compound 13 had activity towards leukemia, renal cancer, non-small cell lung cancer and breast cancer. Furthermore, compounds 16 and 17 exhibited significant degrees of activity towards non-small cell lung cancer and breast cancer (Table 2).
![]()
Figure 1. Common structural features of the active compounds.
![]()
Table 1. The CDK2 inhibitory activity of tested compounds (IC50 in µg/ml).
The data represent means values from three independent experiments plus the standard deviation (SD).
![]()
Table 2. In vitro antitumor activity data of some active compounds.
GI50: concentration giving 50% inhibition; TGI: concentration giving total growth inhibition; LC50: concentration having 50% lethal effect; Δ is considered low if 1, moderate if > 1 and high if ≥ 3; Subpanels showing a statistical measure of differential sensitivity with respect to the indicated response parameters.
4. Conclusion
The synthesized α, β-bifunctional oxygen, sulfur, halogen and nitrogen derivatives of 1,2,4-triazines have shown promising CDK2 activities. Out of eleven active compounds, four of them (11, 13, 16 and 17) have shown very good CDK2 enzyme inhibiting activity. In these compounds N-(amino(hydrazinyl)methyl)hydrazinecarbo- thioamide scaffold was found to be an essential molecular feature in CDK2 enzyme inhibition activity. However these four compounds have also shown significant activity against various tumor cell-lines in subpanel assay.
Acknowledgements
The authors are grateful to King Abdulaziz University for providing research facilities to perform the present research work.
NOTES
*Corresponding author.