1. Introduction
Nanotechnology is one of the leading scientific fields today since it combines knowledge from the fields of Physics, Chemistry, Biology, Medicine and Engineering. The application and use of nanomaterials are extensive such as in electronic and mechanical devices, optical magnetic components, tissue engineering magnetic storage systems and magnetic resonance imaging [1] [2] . Nanotechnology and material technology are new techniques for synthesis and processing manipulation and assembly using natures own building blocks (atoms, molecules or macromolecules) for the intelligent design of functional materials, components and systems with attractive qualities and functions [3] [4] .
Ferrites are well-known magnetic nanomaterials intensively studied as a recording media due to their superior physical properties. These properties make ferrites an ideal candidate for technical applications such as magnetic resonance imaging enhancement, catalysis, sensors and pigments [5] . Mixed spinel ferrites have been studied intensively over the last few years due to their potential applications. Spinel ferrites have the chemical formula MFe2O4 in which M can be any divalent metal cations. In spinel ferrite, oxygen forms face centre cubic (FCC) lattice with divalent cations at tetrahedral (A) and/or octahedral (B) sites. Magnesium ferrite (MgFe2O4) has an inverse spinel structure with the preference of Mg2+ cations mainly on octahedral sites [6] -[9] , while Zinc ferrite (ZnFe2O4) has normal spinel structure, in which Zn2+ cations mainly occupy tetrahedral sites [6] [10] .
The small scale size of the well-known spinel ferrites has opened up the door for intensive research to utilize their properties for biomedical applications [11] -[13] . Numerous methods were reported in literature showing the possibilities of producing particle with size in the range of 2 - 100 nm. Among these methods are co-preci- pitation, hydrothermal and sol-gel Methods [14] [15] , which were reported to be fast and producing high quality nanoparticles.
In this work, Mg1-xZnxFe2O4 nanoferrites where x = 0.0, 0.2, 0.4, 0.6 and 0.8) were synthesized using co-pre- cipitation methods. X-ray diffraction (XRD) was used in order to investigate the structural of Zn substituted magnesium nano-ferrites and to determine the lattice parameters and the space group symmetry. Ultraviolet visible spectrometer (UV vis) and Fourier Transform Infrared Spectroscopy (FTIR) were used to investigate the optical properties of crystallite nanoparticles.
2. Material and Method
Mg Zn ferrite (Mg1-xZnxFe2O4) nanoparticles with composition (x = 0.0, 0.2, 0.4, 0.6 and 0.8) was prepared by the co-precipitation method. Figure 1 shows the synthesis scheme for nanoparticles production. Stoichiometric amounts from pure raw materials of Fe(NO3)3∙9H2O, Mg(NO3)2∙6H2O, Zn(NO3)2∙6H2O, and NaOH were used to prepare the required solutions with required molarities. The solution of Fe(NO3)3∙9H2O, 0.4 M (25 ml), Mg(NO3)2∙6H2O 0.2M (25 ml) and Zn(NO3)2∙6H2O were first mixed and then slowly added 3Molarity of NaOH (25 ml) solution under stirring of 3000 rpm for 30 minutes to obtain a mixture of pH 11 - 13. The colloidal solution was then kept in a water bath at 80˚C for 1 hour to assure removal of NaNO3 from the powder. The produced precipitate was washed 10 times with hot deionized water until the filtrate had a pH 7 [16] [17] . Then the
![]()
Figure 1. Synthesis scheme for nanoparticles production.
samples were dried and grinned to absolute powder and annealed to 450˚C for 6 hours in temperature controlled muffle furnace Vulcan A-550 at a heating rate 10˚C/min.
The XRD analysis was carried out to confirm the purity of the synthesize materials using Shimadzu 6000 X-ray diffractometer with Cu-kα radiation of a wavelength λ = 1.5406 Å source.
FTIR measurements were performed using (Mattson, model 960m0016) spectra, while the absorption of solution with different concentration was calculated using UV min 1240 spectrometer Shimadzy.
3. Results and Discussion
3.1. Crystal Analysis
Determination of the crystal structure, the lattice parameters and the space group symmetry are importance in the study of structural, electrical and optical properties of the nanoparticle ferrites. The information of single phase Mg1-xZnxFe2O4 is confirmed after analyzing the x-ray diffraction pattern by MDI Jade 5.0, ORIGAN and FULLPROF. The crystal structure is found to be cubic with space group Fd3m. The X-ray diffraction (XRD) is carried out at room temperature. Figure 2 shows the X-ray diffraction patterns for the sample MgFe2O4 nanoparticles with x = 0.0, while Figure 3 shows the X-ray diffraction patterns for the samples Mg1-xZnxFe2O4 nanoparticles with different composition (x = 0.2, 0.4, 0.6 and 0.8). The peaks were indexed as (111), (220), (311), (222), (400), (422), (511), (440), and (533). The crystallite size, lattice constants, volume and densities are listed in Table 1. The crystal size is calculated using the Debye-Scherer’s equation [18] :
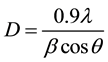 (1)
(1)
![]()
Figure 2. XRD patterns of Mg1-xZnxFe2O4 nano-ferrites for x = 0.
![]()
Figure 3. XRD patterns of Mg1-xZnxFe2O4 nano-ferrites for x = 0.2, 0.4, 0.6 and 0.8.
![]()
Table 1. Particle size (D), Lattice constant (a), Volume and Density of Mg1-xZnxFe2O4 nano-ferrites.
where D is the average crystallite size, q is the diffraction angle, λ is the wavelength of incident X-ray and 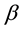 is the full width at half maximum (FWHM) of the (XRD) peak in units of radians. The crystallite size vs. Zn concentration is plotted in Figure 4 and the crystallite size is found to scattered in the range 21 - 42.8 nm for different compositions. In the obtained diffraction pattern the lattice constants are found to increase from (8.09 - 8.44 Å) as the Zn concentration increased. The particle size of the nanocrystalline samples, calculated from the XRD data using Equation (1), are remained within the range (21.0 - 42.8 nm). The increased in lattice constant with Zn concentration may be due to the fact that Zn2+ ions (0.82 Å) is larger than that of the Mg2+ ions (0.72 Å). Addition of Zn2+ at the expense of Mg2+ in the ferrite is expected to increase the lattice constant. The lattice parameter (a) is estimated using lattice spacing (d) values and respective miller indices (hkl). The lattice constant (a) was calculated by MDI Jade 5.0 program and using the equation [19] :
is the full width at half maximum (FWHM) of the (XRD) peak in units of radians. The crystallite size vs. Zn concentration is plotted in Figure 4 and the crystallite size is found to scattered in the range 21 - 42.8 nm for different compositions. In the obtained diffraction pattern the lattice constants are found to increase from (8.09 - 8.44 Å) as the Zn concentration increased. The particle size of the nanocrystalline samples, calculated from the XRD data using Equation (1), are remained within the range (21.0 - 42.8 nm). The increased in lattice constant with Zn concentration may be due to the fact that Zn2+ ions (0.82 Å) is larger than that of the Mg2+ ions (0.72 Å). Addition of Zn2+ at the expense of Mg2+ in the ferrite is expected to increase the lattice constant. The lattice parameter (a) is estimated using lattice spacing (d) values and respective miller indices (hkl). The lattice constant (a) was calculated by MDI Jade 5.0 program and using the equation [19] :
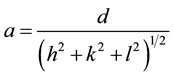 (2)
(2)
where a is the lattice parameter, d is the lattice spacing and h, k, l are the miller indices. The lattice parameter is obtained using XRD data lies in the range of 8.1 - 8.4 Å for different Zn concentration as shown in Figure 5. It increases with increasing Zn concentration due to the larger ionic radius of Zn2+ (0.08 nm) Cation as compared to ionic radius of Mg2+ (0.06 nm) Cation [20] [21] .
3.2. FTIR Analysis
In order to investigate the chemical functional groups on the the synthesized Mg1-xZnxFe2O4, FTIR spectroscopy are performed. The FTIR of nanocrystals powders (as pellets in KBr) in the range of 400 cm−1 to 4000 cm−1 is shown in Figure 6 for pure sample where x = 0. The FTIR of Mg1-xZnxFe2O4 nano-ferrites powders for samples where x = 0.2, 0.4, 0.6 and 0.8 is plotted and shown in Figure 7. The bands (ʋ1, ʋ2) for the samples are found to be in range 3148 - 3450 cm−1 and 1644 - 1649 cm−1, respectively. These observed bands maybe are due to the O-H stretching vibration of the free absorbed water and indicates the existence of hydroxyl groups in the synthesized ferrites, which is observed in previous experiments [22] [23] . The band (ʋ3) for the samples are observed around 1404 cm−1 and is attributed to the C=O stretching vibration of the carboxyl group. In range 1107 - 1147 cm−1, the band (ʋ4) is observed and is related to the stretching vibration due to nitrate group [24] [25] . In the range of 800 - 400 cm−1, two main absorption bands with very low intensity are observed around 400 and 600 cm−1 and may be is caused by metal oxygen vibration in the octahedral side. The ʋ1, ʋ2, ʋ3, ʋ4 and ʋ5 are absorption bands around 3148 - 3450, 1644 - 1649, 1404, 1107 - 1147 and 612 - 663 cm−1, respectively for the samples with different compositions and are attributed to the vibration of the tetrahedral and octahedral metal-oxygen (M-O) bands in the lattices of the synthesized nanocrystals. The FTIR frequency bands for various Zn and Mg contents are listed in Table 2.
3.3. UV Visible Analysis
The absorption as a function of wavelength for sample Mg0.6Zn0.4Fe2O4 is shown in Figure 8. Maximum absorption for the sample is observed at wavelength 232.4 nm. Several models are used to determine the optical properties of nano ferrites. The most widespread is the Tauc model which allows the derive of the band gap energy Eg from (αhʋ)2 as function of the incident energy (hʋ). The Tauc optical gap associated with the Mg1-xZnxFe2O4 nano ferrites is determine through an extrapolation of the linear trend observed in the spectral
![]()
Figure 4. Particle size as a function of Zn concentration of Mg1-xZnxFe2O4 nano-ferrites.
![]()
Figure 5. Lattice parameter as a function of Zn concentration of Mg1-xZnxFe2O4 nano-ferrites.
![]()
Figure 6. The FTIR spectrum of Mg1-xZnxFe2O4 nano-ferrites for x = 0.
dependence of (αhʋ)2 over a limited range of photon energies hʋ. The Tauc optical gap is defined as occurring at the intercept of this linear extrapolation with Y axis. The absorption coefficient α near the band edge in many Nano ferrites shows an exponential upon photon energy usually obeying the relation [26] :
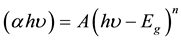 (3)
(3)
where, α is the absorption coefficient and A is known as edge width parameter, Eg is the energy band gap, n = (1/2, 1, 2) is the a constant dependent on the degree of transition, (hʋ) is incident photon energy. The band gap is
![]()
Figure 7. The FTIR spectrum of Mg1-xZnxFe2O4 nano-ferrites for x = 0.2, 0.4, 0.6 and 0.8.
![]()
Figure 8. Absorption as a function of wavelength for sample of Mg0.6Zn0.4Fe2O4 composition.
![]()
Table 2. Wave numbers, wavelength, and energy band gaps of Mg1-xZnxFe2O4 nano-ferrites.
then evaluated by plotting (hʋ) versus (αhʋ)2 and extrapolating the tangent on the X-axis (Tauc plots). Figure 9 shows Tauc plot method for sample of MgFe2O4, and the energy band gap are found to be 4.77, 4.82, 4.86, 4.87 and 4.95 eV for samples with different concentration (x = 0.2, 0.4, 0.6 and 0.8), respectively.
4. Conclusion
Mg1-xZnxFe2O4 nanparticles was prepared successfully using co-precipitation method. The formation of single phase crystallite structure with size in the range 21.0 - 42.8 nm was confirmed by X-ray diffraction. Lattice parameter was found to increase with Zn concentration and this may be due to the larger ionic radius of the Zn2+ ion. FTIR spectrum exhibited expected main absorption bands, thereby confirming the spinel structure. Optical band gap energy Mg1-xZnxFe2O4 nanoferrite was found to be in the range 4.77 to 4.95 eV for samples with different ratio of Mg Zn. The synthesized nanoferrites are expected to be useful in several technological applications such as soft magnets and magnetic fluids for hyperthermia. The structural and properties of spinel ferrites
![]()
Figure 9. Plot of (αhn)2 versus hn for MgFe2O4 nano-ferrites.
depend upon the method of preparation, the nature of substitutional element and the concentration of the substitution element. Attempts can be made to prepare the samples by different methods to get desired properties and crystallite size.
Acknowledgements
Abdalrawf I. Ahmed would like to thank the department of physics particularly material laboratory, Al-neelain University, Sudan for supporting this research.
NOTES
*Corresponding author.