Titrimetric Study of the Solubility and Dissociation of Benzoic Acid in Water: Effect of Ionic Strength and Temperature ()
1. Introduction
Equilibrium studies concerning the solubility and the dissociation process of many well-known weak acids in aqueous solutions have been reported over the years by following many physical and analytical methods. Benzoic acid, C6H5COOH, is one of the simplest organic acids of aromatic series with low solubility in water. Its high water soluble sodium salt (sodium benzoate) is used as food preservative to inhibit the growth of yeasts and moulds. Benzoic acid dissociates partially in water to produce benzoate anion and hydronium cation with an apparent dissociation constant that depends on many factors. It is well-known that the temperature and ionic strength can affect the equilibrium state of weak acids in solution, which is of great importance in chemical and biomedical analysis [1] [2] . Water molecules can attach to benzoic acid by hydrogen bonding and work to stabilizing the formation of the “benzoate” ion. The addition of salts in water gives rise to slight changes in the water-water interactions and that also affects the solute-solute correlations [3] . Benzoic acid dissolves only slightly in cold water though the polarization of carboxylic acid group. The bulk of the benzoic acid molecule has no internal stabilizing structure that favours carboxylate group over carboxylic acid that makes the molecule behave as a weak electrolyte in aqueous solutions [4] .
In this work, the apparent dissociation constants (Kc) of benzoic acid in aqueous solutions have been determined titrimetrically with different values of ionic strengths at a small range of temperatures. For each case the thermodynamic dissociation constant (as pKa) has been evaluated graphically by using equations derived from Debye-Hückel Limiting law, which was improved and used in this work to obtain more accurate results as compared to the reported pKa values in the literature. The thermodynamic parameters of the dissociation process of benzoic acid in water have been reported at standard condition. The absence of such information on benzoic acid in the literature following this method motivated us to carry out this study.
Theory
In a saturated aqueous solution, benzoic acid has a little molar solubility with the following equilibrium:
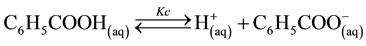 (1)
(1)
where Kc is the apparent dissociation constant of benzoic acid, which is affected by several factors including temperature and ionic strength. The total molar solubility of benzoic acid in water is
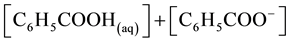
or 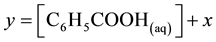 (2)
(2)
where y represents the total molar solubility and x represents the molar solubility of benzoate ion or hydrogen ion in aqueous solution,
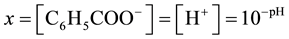 (3)
(3)
Total molar solubility can be determined titrimetrically against a standardized strong base solution.
The equilibrium in Equation (1) can be expressed by two ways
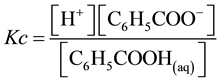 (4)
(4)
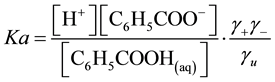 (5)
(5)
where 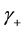 and
and 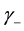 are activity coefficients of the dissociated ions of benzoic acid and
are activity coefficients of the dissociated ions of benzoic acid and 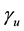 is that for undissociated benzoic acid; Ka is the thermodynamic dissociation constant at infinite dilution at a given temperature [5] .
is that for undissociated benzoic acid; Ka is the thermodynamic dissociation constant at infinite dilution at a given temperature [5] .
From Equations (4) and (5)
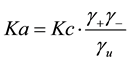 (6)
(6)
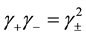 , where
, where 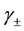 is the mean activity coefficient of the dissociated ions and is affected by ionic strength of the solution, while
is the mean activity coefficient of the dissociated ions and is affected by ionic strength of the solution, while 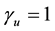 is for undissociated benzoic acid in solution.
is for undissociated benzoic acid in solution.
![]() (7)
(7)
where I is the ionic strength; ci is the molar concentration of ion of ith type; and zi is its charge number of ith type in solution. In this work the contribution of benzoic acid to ionic strength (I) values is ignored due to its relatively small dissociation. Sodium chloride is used in this work as an electrolyte to raise the ionic strength in each of particular solution of benzoic acid and the value of ionic strength (I) for each solution is the molar concentration of NaCl.
![]() is related to ionic strength (I) by the following law:
is related to ionic strength (I) by the following law:
![]() (8)
(8)
where B is a quantity that depends on some physical properties of the solution such as dielectric constant and temperature [5] .
Equation (6) and Equation (8) give
![]() (9)
(9)
![]() (10)
(10)
For aqueous solutions at 25˚C, Equation (10) becomes
![]() (11)
(11)
2. Experimental
All chemicals used in this study were of analytical reagent grade and were used without further purification. The solvent used throughout was water, which was three times distilled. Carbonate-free NaOH solutions were prepared as suggested by Vogel [6] .
In a typical preparation for the solutions, 1 g of benzoic acid was placed in each of six dry 250 ml stoppered bottles. In each bottle, 100 ml NaCl solution prepared by 100 ml volumetric flask of concentrations 0.00, 0.050, 0.10, 0.30, 0.40, and 0.50 M were poured in these bottles and then put in a thermostat at specific temperature with shaking vigorously in the beginning, and then occasionally for two hours. From each bottle 20.0 ml was withdrawn by 20.0 ml pipette with filter to prevent drawing small solids into the pipette, then discharged to 250 ml conical flask after removing the filter. The concentration of benzoic acid in each solution was determined by titration with 0.050 M NaOH. The pH of each solution in the bottles was measured by using 3 decimal digits calibrated pH-meter at the different temperatures used. Class “A” calibrated volumetric glassware were used in this work.
3. Results and Discussion
3.1. Effect of Salts on the Solubility and the Apparent Dissociation Constant at 25˚C
The influence of the ionic strength on the solubility and the apparent dissociation constant (Kc) of benzoic acid at 25˚C was examined at six different ionic strengths in the range of 0.0013 - 0.50 M by adding NaCl. The solubility of benzoic acid in water for each concentration of NaCl was evaluated titrimetrically, followed by measuring the pH of each solution. The experimental data are presented in Table 1, which taken as average of several results of measurements that show high precision. The observed solubility (from the amount of sodium hydrox-
![]()
Table 1. Effect of NaCl concentration on the solubility of benzoic acid in water at 25˚C.
ide solution) and pH values of benzoic acid in water related inversely with NaCl concentrations. For each solution, the apparent dissociation constant (Kc) of benzoic acid was evaluated by using the following modulated expression that derived from Equation (4) and from the expression of finding pH value:
![]() (12)
(12)
where y is the total molar solubility of benzoic acid in solution ![]() that could be evaluated from the following expression:
that could be evaluated from the following expression:
![]() (13)
(13)
where VNaOH, MNaOH, and VBA are respectively; volume of sodium hydroxide per liter, molarity of sodium hydroxide per mol/L, and volume of benzoic acid sample per liter. Table 2 contains values of y, 10−pH, Kc, and ionic strength for each benzoic acid solution at 25˚C. Figure 1 shows the plot of (logKc) as the y-axis versus ![]() as the x-axis, which illustrates direct and linear relationship after applying the linear least-squares analysis of the data (r2 = 0.99188). The value of the actual thermodynamic dissociation constant, Ka, of benzoic acid at 25˚C can be obtained by extrapolation to zero ionic strength. The value of estimated logKa from the plot is −4.176 ± 0.01035, which its inverse (4.176) represents pKa of benzoic acid at 25˚C. This value is in a good agreement with reported values in the literature from using different methods; electrophoresis 4.12 [7] , potentiometry 4.20 [8] , spectrometry 4.19 [9] , and liquid chromatography 4.18 [10] .
as the x-axis, which illustrates direct and linear relationship after applying the linear least-squares analysis of the data (r2 = 0.99188). The value of the actual thermodynamic dissociation constant, Ka, of benzoic acid at 25˚C can be obtained by extrapolation to zero ionic strength. The value of estimated logKa from the plot is −4.176 ± 0.01035, which its inverse (4.176) represents pKa of benzoic acid at 25˚C. This value is in a good agreement with reported values in the literature from using different methods; electrophoresis 4.12 [7] , potentiometry 4.20 [8] , spectrometry 4.19 [9] , and liquid chromatography 4.18 [10] .
The mean activity coefficient of the dissociated ions ![]() could be calculated for each solution from the following equation that derived from Equation (9):
could be calculated for each solution from the following equation that derived from Equation (9):
![]() (14)
(14)
Plotting ![]() as the y-axis versus
as the y-axis versus ![]() as the x-axis (Figure 2) gives rise to a straight line with a limiting
as the x-axis (Figure 2) gives rise to a straight line with a limiting
slope equal to −0.2698. The value of mean activity coefficient for any concentration of sodium chloride in the range used can be estimated from this plot.
![]()
Figure 1. The effect of ionic strength on the values of acid dissociation constant (Kc) of benzoic acid in water at 25˚C.
![]()
Figure 2. The effect of ionic strength on the values of the mean activity coefficient ![]() of benzoic acid in water at 25˚C.
of benzoic acid in water at 25˚C.
![]()
Table 2. Effect of ionic strength on the value of acid dissociation constant of benzoic acid in water at 25˚C.
3.2. Effect of Temperature on the Equilibrium Dissociation Constant
By repeating the same procedure (as in the last section) six more times with temperature values around 25˚C, the solubility of benzoic acid in water for the same concentrations of NaCl used at 25˚C, was evaluated titrimetrically at temperatures between 16˚C and 41˚C, followed by measuring the pH of each solution (the average of the results that show high precision at each temperature was taken for NaOH volume and pH values). At each temperature, Kc value for each NaCl concentration used was calculated by the same method as done in the case of 25˚C, and the thermodynamic dissociation constant (as pKa) was also estimated from plots of logKc vs ![]() for each temperature. In thermodynamics the use of molarity is often not convenient, because the volume of most solutions slightly depends on temperature due to thermal expansion. This problem was resolved by introducing temperature correction to maintain the same NaCl concentration for all temperatures used, such correction was worked by testing the increase or decrease in solution volume of NaCl inside the 100 mL volumetric flask at each selected temperature relative to the standard volume of the flask at 20˚C, then preparing the NaCl solution with a starting volume more or less than 100 mL to be exactly 100 mL when attained thermal equilibrium inside the thermostat with the selected temperature. Figure 3 illustrates the relation between the estimated pKa
for each temperature. In thermodynamics the use of molarity is often not convenient, because the volume of most solutions slightly depends on temperature due to thermal expansion. This problem was resolved by introducing temperature correction to maintain the same NaCl concentration for all temperatures used, such correction was worked by testing the increase or decrease in solution volume of NaCl inside the 100 mL volumetric flask at each selected temperature relative to the standard volume of the flask at 20˚C, then preparing the NaCl solution with a starting volume more or less than 100 mL to be exactly 100 mL when attained thermal equilibrium inside the thermostat with the selected temperature. Figure 3 illustrates the relation between the estimated pKa
![]()
Figure 3. The effect of temperature on the value of the thermodynamic dissociation constant (Ka) of benzoic acid in water.
values and temperatures used. In the Figure, the values of pKa are inversely proportional with temperature between 16˚C and 30˚C. In contrast the values of pKa are directly proportional with temperature between 30˚C and 41˚C, i.e. no regular correlation between pKa of benzoic acid and range of temperatures that used. From the experimental data; the amount of NaOH used in titration and the value of pH for each temperature used, the solubility of benzoic acid in water is directly proportional with temperature, and the capability of the benzoic acid molecule to dissociate is not always increases as temperature increases.
The values of enthalpy change ![]() and entropy change
and entropy change ![]() of the dissociation process of benzoic acid in the ordinary temperature range between 16˚C and 30˚C were obtained according to the following linear thermodynamic equation (Van’t Hoff equation):
of the dissociation process of benzoic acid in the ordinary temperature range between 16˚C and 30˚C were obtained according to the following linear thermodynamic equation (Van’t Hoff equation):
![]() (15)
(15)
where R is the molar gas constant (8.314 J∙K−1∙mol−1), and taken into account that ![]() and
and ![]() are independent on temperature due to the relatively small change in temperature values. A plot of log(Ka) as y-axis against 1/T as x-axis gives
are independent on temperature due to the relatively small change in temperature values. A plot of log(Ka) as y-axis against 1/T as x-axis gives ![]() and
and ![]() values. Gibbs free energy
values. Gibbs free energy ![]() was calculated from the following thermodynamic relation:
was calculated from the following thermodynamic relation:
![]() (16)
(16)
Figure 4 shows the plot of Equation (15) for benzoic acid in water at the temperature range between 16˚C and 30˚C. A linear least-squares analysis of logKa vs. 1/T data illustrates a linear relationship (r2 = 0.99237) and gave the following results for the standard values of the thermodynamic parameters for the dissociation of benzoic acid in water:![]() ,
, ![]() , and
, and![]() .
.
Although the details of a dissociation process are not usually revealed by a thermodynamic study, a conclusion can be drawn from the above results that the dissociation process represented by Equation (1) is nonspontaneous and endothermic. It is clear that both the enthalpy change and the entropy change make benzoic acid molecule to be not favoured to dissociate in water between 16˚C and 30˚C. These two thermodynamic parameters; positive enthalpy change and negative entropy change contribute in retarding the dissociation process making Gibbs energy change to be positive, therefore the dissociation process of benzoic acid molecule is non-
![]()
Figure 4. Plot of Equation (15) for benzoic acid in water.
spontaneous. Moreover, the negative entropy change means that benzoic acid in water attain a more ordered state after its dissociation process.
At a temperature higher than 30˚C, benzoic acid in water behaves differently. As a temperature increases the capability of benzoic acid to dissociate decreases and that leads to reduce the value of Ka. The process in this range is certainly exothermic and compatible with Le Chatelier’s principle [5] . The effect of temperature on the acidity strength of benzoic acid is related to inductive effect inside the molecule, which is practically observable effect of the move of charge through atoms in benzoic acid molecule resulting in a continual state of bond polarization [11] . Over 30˚C, benzoic acid molecule behaves less acidic due to the decrease in the effect of electron releasing group inside the benzoic acid molecule on the acidic hydrogen as observed from its numerical values of pKa at different temperatures.
4. Conclusion
The observed solubility and pH values of benzoic acid in water related inversely with NaCl concentrations. The calculated apparent dissociation constant (Kc) is directly related. No regular correlation is between the thermodynamic dissociation constant of benzoic acid and temperatures between 16˚C and 41˚C. The dissociation process of benzoic acid molecule around 25˚C is non-spontaneous.
Acknowledgements
The help in the arrangement of equations by Prof. Khalid Tawarah from Yarmouk University-Irbid is highly appreciated.