Mycotic Aneurysm of Infrarenal Aorta: A Case Report and Review of Literature ()
1. Case Report
A 56-year-old gentleman, presented with a history of fever for 3 weeks, abdominal pain radiating to the right testis and lower back pain also had loss of appetite, nausea and vomiting. A consult to general practitioner was made few days prior to admission and was able to complete course of oral antibiotic, without remarkable improvement.
Systemic review was otherwise unremarkable. Patient is a smoker without other significant past medical history.
On physical examination, patient had temperature of 38.9˚C. Abdominal assessment showed tenderness on the right hypochondriac region and positive right renal punch. Testicular examination was unremarkable. Cardiopulmonary status was normal.
Preliminary investigations showed elevated white cell count of 23.7 × 109 cells/l and Serum ALP 246 U/L. Urine dipstick was positive to nitrite and leukocytes. Urine cytology yielded an acute infective result. Serum sodium of 127 mmol/L and albumin of 27 g/L. Coagulation profile were unremarkable. Initial blood cultures were negative.
Patient was admitted to our hospital with an initial impression of acute pyelonephritis. Given the history of penicillin allergy, IV Ciprofloxacin 400 mg was commenced 12-hourly. However, imaging of kidneys showed no abnormality.
On the second hospital day, patient had persistent fever, abdominal and low back pain, hence, Computed Tomography scan of the Abdomen and Pelvis was ordered which revealed hepatic abscess, and a small saccular aneurysm arising from the left anterolateral aspect of infrarenal abdominal aorta, with surrounding thrombus formation and inflammatory stranding suggestive of Mycotic Aneurysm (Figure 1(a) and Figure 1(b)). Metronidazole 500 mg IV for liver abscess was added. Attempted ultrasound guided aspiration of liver abscess was performed, however, did not yield any fluid.
C-reactive protein was 227 mg/dL. Septic screening, including urine and and repeated blood cultures at a different interval, syphilis RPR and HIV test were all negative. Echocardiogram did not establish evidence of Infective Endocarditis. Other investigations conducted include ultrasound of testes, which demonstrated Right epididymo-orchitis, hence Oral Doxycycline was started.
On the fifth day of admission, fever persisted and abdominal pain became intractable. Inflammatory markers such as C-reactive Protein and white cell count continued to rise despite the antibiotics. Computed Tomography Aortogram was done which demonstrated interval increase in size of the saccular infrarenal mycotic aneurysm with more prominent inflammatory changes extending inferiorly to origins of both common iliac arteries and also around the origin of the inferior mesenteric artery (Figure 2(a) and Figure 2(b), Figure 3(a) and Figure 3(b)).
Immediately referred to vascular surgery and underwent emergency Percutaneous Endovascular Repair (EVAR) with Covered Endovascular Revascularization of Aortic Bifurcation (CERAB) and glue embolisation of the aneurysmal sac.
Bilateral percutaneous incisions were performed on the groin. Sheaths were inserted using guidewire through transfemoral approach. At renal arteries level, aortogram was performed and microcatheter was placed in aneu- rysm sac. Balloon expandable covered stent was deployed just above the bufircation of the abdominal aorta. Another stent was deployed just distal to the most distal renal accessory artery with good overlap between the two aortic stents. Afterwhich, proximal atrium was overinflated with balloon.
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) and (b) Interval increase in size of the saccular aneurysm seen arising from the left anterolateral aspect of the infrarenal abdominal aorta is seen, measuring 4.8 × 4.5 × 5.9 cm (previous 2.1 × 1.8 × 1.0 cm). A rim of surrounding thrombus formation is again seen. The upper margin of the aneurysm is about 5.0 cm from the origin of the left renal artery and it extends inferiorly to just above the aortic bifurcation. The surrounding thickening and enhancement of the aortic walls with associated periaortic stranding appears more prominent and extends inferiorly to around the origins of both common iliac arteries and also around the origin of the inferior mesenteric artery.
![]()
![]() (a) (b)
(a) (b)
Figure 1.(a) and (b) A 2.1 × 1.8 × 1.0 cm saccular aneurysm is seen arising from the left anterolateral aspect of the infrarenal abdominal aorta. A surrounding rim of thrombus formation is seen. The upper margin of the aneurysm is seen approximately 5.3 cm from the origin of the left renal artery. Inferiorly, the aneurysm ends before the aortic bifurcation. There is subtle thickening and enhancement of the aortic walls most notably at the level of the aneurysm. Periaortic stranding and multiple small volume para-aortic lymph nodes are seen, likely reactive in nature. Mild circumferential fat stranding is also seen around the proximal common iliac arteries. No periaortic gas or fluid collection is detected. The origin of the inferior mesenteric artery is amidst the inflammatory changes, and is markedly attenuated.
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) and (b) Interval increase in size of the saccular aneurysm seen arising from the left anterolateral aspect of the infrarenal abdominal aorta is seen, measuring 4.8 × 4.5 × 5.9 cm (previous 2.1 × 1.8 × 1.0 cm). A rim of surrounding thrombus formation is again seen. The upper margin of the aneurysm is about 5.0 cm from the origin of the left renal artery and it extends inferiorly to just above the aortic bifurcation. The surrounding thickening and enhancement of the aortic walls with associated periaortic stranding appears more prominent and extends inferiorly to around the origins of both common iliac arteries and also around the origin of the inferior mesenteric artery.
![]()
![]() (a) (b)
(a) (b)
Figure 3. (a) and (b) Contrast enhanced CT images of the abdomen and pelvis demonstrating the saccular aneurysm seen from the left infrarenal abdominal aorta.
Decision was made to perform CERAB procedure, as distal aortic seal was needed and left common iliac dissection occurred post aortic stent deployment. Bilateral common iliac artery kissing stents deployed simultaneously to raise the aortic bifurcation. On aortogram, there was minimal flow in the sac which drained into median sacral artery, hence Lipiodol and glue mixture was used to fill the aneurysm sac through the microcatheter with good result. Prior to closure, aortogram revealed no further flow into the sac and good filling of the iliac arteries. After the procedure, peripheral pulses were palpable. The procedure was uneventful without post-oper- ative complication. White cell count and CRP improved to normal. (Graph 1)
Symptoms of fever, abdominal and back pain disappeared. Repeated imaging of Hepato-Biliary system and Testes showed resolving abscess in the liver and near complete resolution of the right epididymo-orchitis. Patient was discharged after fourteenth hospitalization day with 6 months duration of Ciprofloxacin and 3 months of Metronidazole.
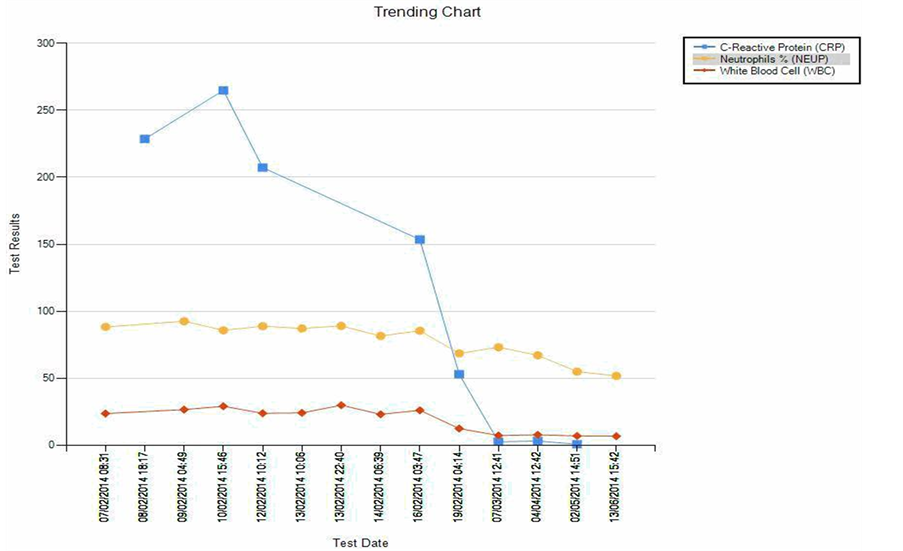
Graph 1.
Four months later, repeated abdominal CT aortogram appeared to have smaller aneurysmal sac without endoleak or para-aortic abscess, whereas the liver abscess showed near complete resoulution. To date there were no documented complications and recurrence of symptoms.
2. Discussion and Literature Review
Aneurysms of the infra-renal aorta are by far the most common arterial aneurysms encountered in clinical practice today: they are three to seven times more common than thoracic aneurysms and affect four times as many men as women [1]. A mycotic aneurysm of the aorta and adjacent arteries is a dreadful condition, threatening life, organs, and limbs [2]. It carries a high risk of complications, especially those with underlying sepsis and extensive periaortic infection. Symptoms are frequently minimal during the early stages and a high index of suspicion is essential to make the diagnosis.
Mycotic aneurysm results from systemic bacteremia and embolization of infectious material, which cause superinfection of a diseased and roughened atherosclerotic plaque acting as a culture media. These emboli usually lodge at the sites of arterial division particularly in the femoral and superior mesenteric arteries. Rarely, organisms may colonize the intact vascular wall through the vasa vasorum, where the arterial wall is weakened by a local suppurative process which results in aneurysm formation [3].
Risk factors include: 1) Endothelial damage caused by atherosclerosis including pre-existing aneurysm 2) Antecedent infection including bacteremia, which appears to be similar in our case having hepatic abscess 3) Arterial injury including iatrogenic mechanisms, such as percutaneous coronary intervention [4]. In a retrospective study conducted by Spelman et.al nearly half of 43 patients with an infected aortic aneurysm were found to have an antecedent infection that included pneumonia, cholecystitis, urinary tract infection, endocarditis, diverticulitis, soft-tissue infection, and osteomyelitis [5].
Mycotic aneurysms are defined by the presence of two or more of the following features: sepsis (fever, leukocytosis and pain), positive blood culture, positive culture from the aneurysmal wall, or characteristic radiological appearance (including irregular aortic wall, rapid growth rate, or saccular appearance of the aneurysm). Negative blood cultures and absence of fever does not exclude the diagnosis when the patient has presented with signs of infection and had characteristic radiological findings but had already been commenced on antibiotics [6].
In Jarrett’s series, only 53% of patients presented with a palpable aneurysm, and less than 50% had aortic calcification. Abdominal pain was present in only 35% of patients. Anderson et al. emphasized that pain, temperature, and fever associated with a pulsatile mass should suggest the diagnosis of mycotic aneurysm. In the series of Mundth et al., pain and fever were present in 94%, with a leukocytosis > 10,000 in 77% of patients and a palpable aneurysm in 65% [7]. Bennett and Cherry emphasized that the one common denominator is the presence of fever > 38.8˚C in the majority of patients [8].
The diagnosis is based upon imaging. CT angiography is the most useful for diagnosing infected aneurysm. MR angiography is an alternative diagnostic study when intravenous contrast is contraindicated. Saccular aneurysm, rapid enlarging of the original aneurysm, and perianeurysm soft tissue mass with or without localized stranded fluid were reported to be typical radiographic manifestations of mycotic aneurysms which appeared to be similar in this case [5]. On laboratory examination, an increased white blood cell count is found in 64 to 71 percent of patients. Inflammatory markers, including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are generally elevated [9].
Early diagnosis and a combination of surgical intervention and prolonged antibiotic therapy are essential for successful treatment. The choice of antibiotic depends upon the organism grown from blood cultures. However, if no organism is grown then the choice should include a broad-spectrum antibiotic treatment with a combination of ceftriaxone, a fluoroquinolone, and piperacillin-tazobactam [1]. The required duration of antibiotic therapy is not well established; recommendations range from 6 - 8 weeks to lifelong [10]. In our case, postoperative antibiotics was administered for at least 3 months and discontinued when patient reveals no further signs of infection.
Prior to the advent of recent less invasive procedure termed endovascular aortic aneurysm repair (EVAR), the gold standard of treatment was wide surgical debridement and in-situ or extra-anatomical repair, however mortality rates of up to 40 per cent are associated with open surgical repair [6] [10]. EVAR has offered an alternative to conventional surgical practice. EVAR most commonly performed for surgical treatment of infected aortic aneurysms, was introduced during 1990’s. It is a minimally invasive approach that involves transfemoral or transiliac placement of a endoluminal graft within the aneurysm, with the goal of completely excluding the aneurysmal sac from the circulation [11]. The endoluminal graft is anchored in place by self-expanding or balloon expandable stents, which may support all or part of the graft. The advantages of this technique are principally related to the absence of surgical exposure of the aorta and avoidance of aortic cross clamping, which are both mandatory during direct graft replacement.
It is recognized as an alternative to open surgery with the anticipation that minimally invasive endovascular procedure may reduce the risk of cardiopulmonary, neurological and renal complications in critically ill patients [12]. The less invasive nature of endovascular aneurysm repair therefore has the potential to reduce the mortality and morbidity of conventional aortic procedures and may offer an opportunity to treat patients with severe coexistent pathologies, who are denied conventional aneurysm repair [13].
Occasionally, EVAR may fail to exclude blood flow from the aneurysmal sac completely, thus may cause endoleak, hence to provide seal on distal aorta, Covered Endovascular Reconstruction of Aortic Bifurcation or CERAB can be performed. Two iliac covered stent-grafts are then placed in this segment, in a “kissing-stent” configuration and inflated. Both stents will make a very tight combination with the aortic stent, as were they moulded together, simulating a new bifurcation. CERAB is safe and feasible and can be performed completely percutaneous. Distal peripheral outflow needs to be sufficient enough be used for the treatment of recurrent or in-stent disease. It is even feasible to treat lesion that extend to the iuxta and/or supral renal aortic region. In a study performed by Peter et al. larger population, longer follow-up, further haemodynamic investigation is needed [14].