Differentiation of Mesenchymal Stem Cells into Hepatocyte-Like Cells by Autologous Serum after Radiofrequency Ablation of Liver Tumor ()
1. Introduction
Expanded bone marrow-derived MSCs possess the potential to differentiate into hepatocyte-like cells [1] -[3] and can therefore be used in cell-based therapy for various liver diseases [4] . In vitro expansion of MSCs has mainly been achieved in the presence of 30% FCS. To date, intravenous infusion of such cells has been considered relatively safe. However, unfavorable immune responses toward FCS have been reported, [5] and these responses are possibly due to contamination from proteins in the serum. Other possible risks of FCS include viral or bacterial infections or prion infection [6] . For these reasons, alternative culture protocols that replace FCS with autologous serum have been suggested.
Although the detailed mechanisms that govern the differentiation of MSCs remain unclear, there is accumulating evidence that bone MSCs may participate in liver regeneration after partial hepatectomy or CCl4 injury [7] . Various growth and differentiation factors, such as cytokines in serum, may play an important role in this differentiation process [8] .
During the past few years, RFA has been applied successfully in the field of surgical oncology. RFA offers an alternative local treatment to in-operable hepatic tumors, and its therapeutic effects, including a 5-year survival rate, are similar to that of the partial hepatectomy [9] . This indicates that bone MSCs obtained after RFA therapy of liver tumors may participate in liver regeneration.
In the present study using rabbits, we investigated the potential of bone MSCs to differentiate into hepatocyte-like cells under the direct influence of autologous serum obtained after RFA therapy of liver tumor.
2. Materials and Methods
2.1. Experimental Animal
Eight to twelve week-old male New Zealand White rabbits with a body weight of 1.5 - 2.0 kg were obtained from the Laboratory Animal Unit of Xi’an Jiaotong University (Certificate No. shannxi 08-005). All animals used in this study were housed in a climate-controlled (21˚C) room under a 12-h light-dark cycle and were given tap water. All operations were performed under general anesthesia using sterile surgical technique and all animals received humane care in accordance with the principles and guidelines prepared by the National Institutes of Health, China.
2.2. Radiofrequency Oncotherapy in a Transplanted Liver Tumor Model and Collection of Autologous Serum
Under aseptic conditions, animals were anesthetized with 40 mg/kg ketamine and 12.5 mg/kg chlorpromazine, and their abdomens were dissected to expose the liver. A 1 mm3 piece of VX2 tumor was surgically implanted into Glisson’s capsule and the wound was closed. After three weeks, an ultrasound was performed on the inoculated area to verify the establishment of the transplanted liver tumor. RFA oncotherapy was performed on the animals according to the method of Merkle EM [10] . The radiofrequency current was delivered by using a Radiofrequency Generator 2000 and a 10 Tsim anchor-shaped electrode (Radio TherapeuticTM Corporation, Mountain View, CA, USA). RFA was performed by placing the electrode needle at the center of the hepatic tumor and expanding outward to a diameter of 10 - 20 mm to inactivate the hepatic tumor and 40% of hepatic tissues. During the procedure, the applied current, power output, and tissue impedance were monitored constantly. After RF exposure, the cooling system was stopped to measure the local tissue temperature. When the temperature exceeded 60˚C, the ablation was considered adequate. At the end of the procedure, the generator was reactivated while the RF electrode was withdrawn to ablate the needle track and prevent tumor seeding and the wound was closed. Serum samples were collected from rabbits immediately before and 72 hours after radiofrequency therapy of the liver tumor. Whole blood was collected from arteria carotis into 10 ml serum monovettes containing beads coated with kaolin clotting activator and stored at 4˚C overnight. Subsequently, the blood was centrifuged at 3000 rpm for 5 minutes and the upper layer of the serum sample was aliquoted and stored at −20˚C for future analysis.
2.3. Isolation and Culture of Bone MSCs
MSCs were isolated from the rabbit bone marrow aspirates according to the method of Lennon DP [11] . New Zealand white rabbits were intravenously anesthetized via the ear vein with 2-chlorocyclohexanone (40 mg/kg). 5 ml of bone marrow was collected from the tibia using a 12-gauge puncture needle and l0-ml syringe (containing 3000 μg/ml heparin 0.2 ml). The bone marrow was then added to a tube containing the same volume of lymphocyte separation medium (1.073 g/ml, Hyclone Laboratories, Logan, UT, USA) and centrifuged at 1500 rpm for 20 minutes. The ivory-white nuclear cell layer was drawn out and inoculated in 25 ml culture bottles at a cell density of 1 × 106 cells/ml. After the differential adherence method was used to discard the non-adherent cells, the adherent cells were digested by parenzyme and cultured in DMEM (Hyclone Laboratories, Logan, UT, USA) containing: 1 g/l glucose, 1% penicillin/streptomycin, and either selected 30% FCS (Hyclone Laboratories, Logan, UT, USA) (FCS group), 30% autologous serum (AS group), or 30% autologous serum after radiofrequency ablation of the liver tumor (ASRF group) in 12-well plates at a density of 1 × 106 cells per well at 37˚C in a humidified atmosphere containing 5% CO2. After three days, non-adherent cells were washed out and the adherent MSCs were further expanded with the medium described above and fed every three days. At 80% confluency, cells were trypsinized and seeded at low density for further expansion.
2.4. Morphological and Microstructural Observation by Electron Microscopy
After 7 and 14 days of culture, cells were washed with PBS (pH 7.5), fixed in 2.5% glutaraldehyde in 100 mm sodium cacodylate buffer for 48 hr, post-fixed in 1% osmium tetroxide, dehydrated in graded alcohols, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed using a Zeiss EM 9-A Electron Microscope (Zeiss, Oberkochen, Germany).
2.5. Analysis of Cell Cycle
The cell cycle was assessed by flow cytometry after 14 days of culture according to the method of Hulspas R. et al. [12] . Cells were harvested by trypsin digestion, washed with PBS, fixed in 70% alcohol, and re-suspended in 1.0 ml hypotonic PI solution in polypropylene tubes. The PI fluorescence of individual nuclei was measured by BD FACSCalibur Flow Cytometer (Becton Dickinson, Heidelberg, Germany) with an argon laser (488 nm) and emission filter 585 ± 42 nm. For each analysis, 10,000 events were recorded.
2.6. Detection of Markers of Hepatocytes with Immunofluorescence
MSCs were cultured on coverslips in six-well plates until confluent and washed three times with normal saline, 2% saponin/PBS and PBS (pH 7.2), then fixed with paraformaldehyde and treated with 0.1% Triton X-100. Cells were then incubated overnight at 4˚C with primary antibody (1:100, Boster Company, Wuhan, China) against albumin and CK18. After washing with PBS, the cells were then incubated with FITC-conjugated and TRITC-conjugated goat anti-rabbit IgG (Boster Company, Wuhan, China) at 37˚C for 1 hr. Immunofluorescence staining was viewed with a Leica TCS/SP2 laser scanning confocal microscope (Leica, Heidelberg, Germany) and Imaris.v4.0.6 software (Bitplane, Washington DC, USA). The excitation and emission wavelengths for FITC/TRITC were 492 nm and 520 nm, respectively. To exclude the possibility that the trafficking of proteins was affected by the GFP fusion itself, immunofluorescence staining was conducted as previously described [13] .
2.7. Statistical Analysis
All results were expressed as means ± SD. The data was analyzed for statistical significance using Student’s t-test. A P-value of less than 0.05 was considered significant.
3. Results
3.1. Morphology and Microstructure
After seven days in culture in the 30% autologous serum obtained after radiofrequency ablation (ASRF group), most of the spindle-shaped MSCs were rounded and resembled hepatocyte-like cells, whereas such a change was not observed in the cells cultured in 30% fetal calf serum (FCS group) or in 30% rabbit autologous serum (AS group) (Figure 1(a)). Fourteen days after culture, expanded MSCs in the FCS group showed the general subcellular morphological characteristics of normal MSCs (Figures 1(a)-(d)). Most nuclei of these cells contained euchromatin and 1 - 2 nucleoli. As frequently seen in malignant cells, the nuclei had indentations and few lipid droplets present, and all cell organ elles (including lysosomes and mitochondria) were noticeably small (Figure 2(a)). Few or no slender microvilli, cell-cell junction structures or cholangiole were observed on the cell surface (Figure 2(b)). The morphological characteristics of MSCs in AS group were similar to those in the FCS group (Figure 2(c) & Figure 2(d)). In contrast, MSCs cultured in ASRF group showed subcellular morphological
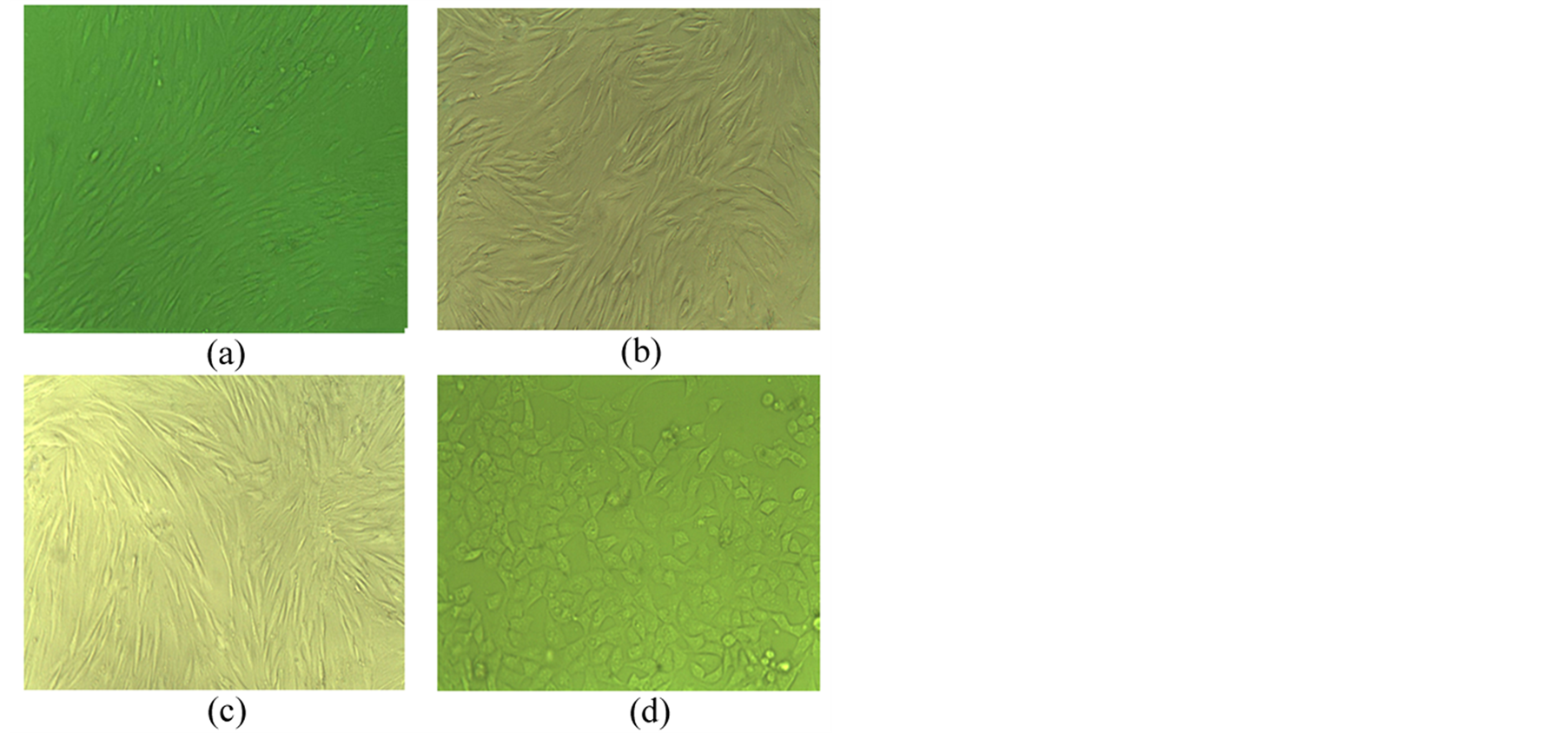
Figure 1. (a) P0 MSCs in 1 week. Original magnification 10×; (b) P4 MSCs in FCS group 2 weeks. Original magnification 20×; (c) P4 MSCs in AS group 2 weeks. Original magnification 20×; (d) P4 MSCs in ASRF group 2 weeks. Original magnification 20×.
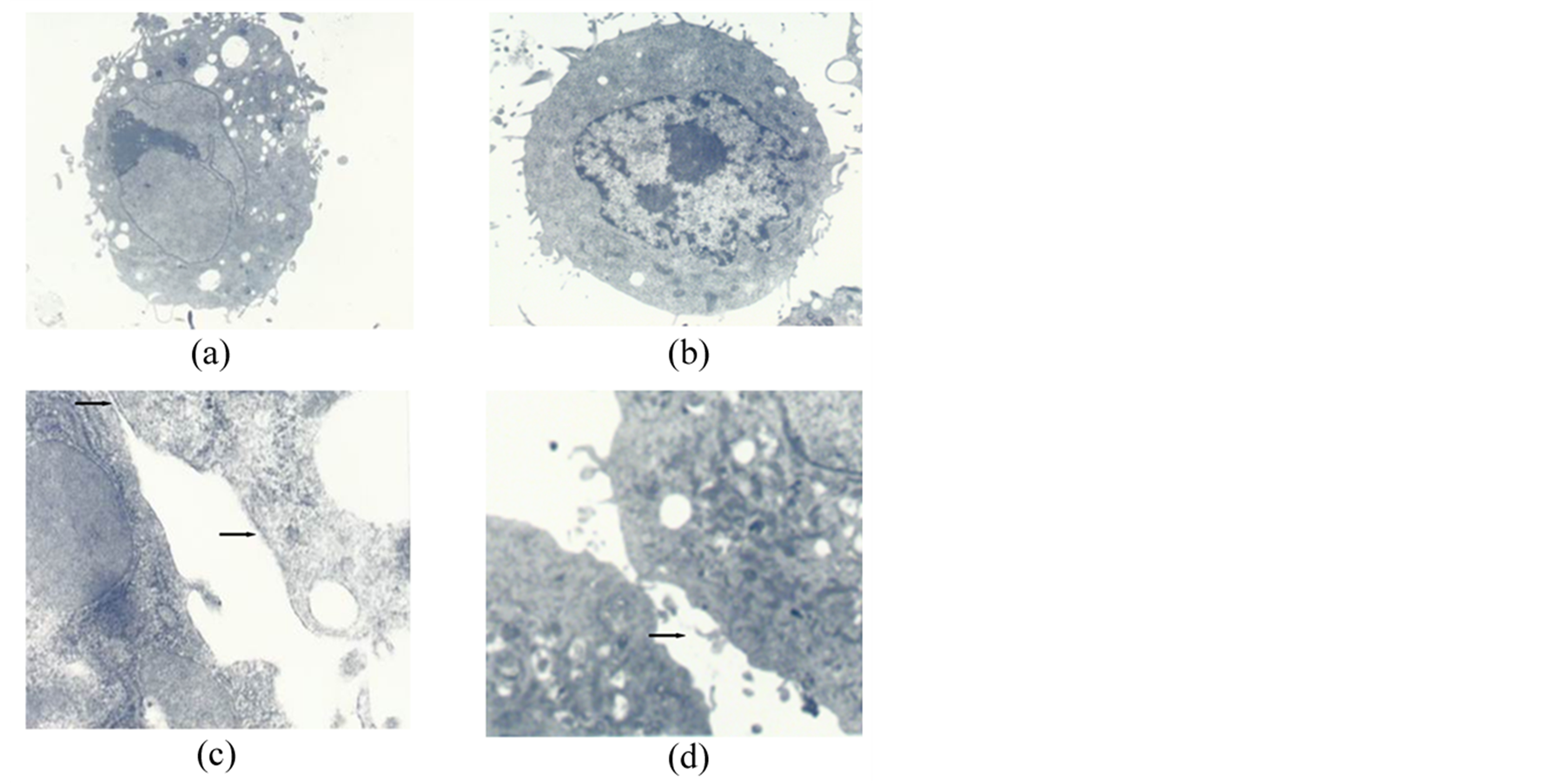
Figure 2. MSCs cultured for 14 days. (a) The morphological characteristics in the FCS group. Original magnification 3500×; (b) The morphological characteristics in the AS group. Original magnification 5000×; (c) Slender microvilli, cell-cell junction structures and cholangiole emerged in the FCS group (arrows). Original magnification 30,000×. MSCs cultured in AS for 14 days; (d) Slender microvilli, cell-cell junction structures in the AS group (arrows). Original magnification 25,000×.
characteristics similar to hepatocytes: the MSC’s karyoplasmic ratio diminished and had focus tendencies (Figure 3(a)). Free ribosomes (Ri) could be seen dispersed in the cell sap and rough endoplasmic reticulum (RER). Also, larger lysosomes and numerous lipid droplets existed, and mitochondria (Mi) increased (Figure 3(b)). Interestingly, cells with typical apoptotic nuclear structures were also occasionally observed in this group. Such cells showed increased cell kytoplasm concentration and electron densities, as well as broken nuclei and the emergence of apoptotic-bodies (Figure 3(c), arrows). Several slender microvilli, cell-cell tight junction (Tj) structures and cholangiole could be seen on the cell surface (Figure 3(d)).
3.2. Cell Cycle Analysis
It is apparent from these values that the cell cycle phase distribution of MSCs in the ASRF group is significantly higher than that of the FCS and AS groups (P < 0.01 for % S/G2/M, G0/G1, and % Apoptosis), whereas no significant difference in the cell cycle distribution was found between the FCS and AS groups. See Table1
3.3. Immunofluorescence Characterization
Indirect immunofluorescence staining for albumin with secondary FITC anti-mouse antibody (green) and for CK18 with secondary TRITC (red) antibody was visualized with a Leica TCS SP2 confocal microscope. Expression of albumin and CK18 could only be observed in the differentiated hepatocyte-like cells in the ASRF group (Figure 4(a) & Figure 4(b)), whereas no immunofluorescence staining for albumin and CK18 was observed
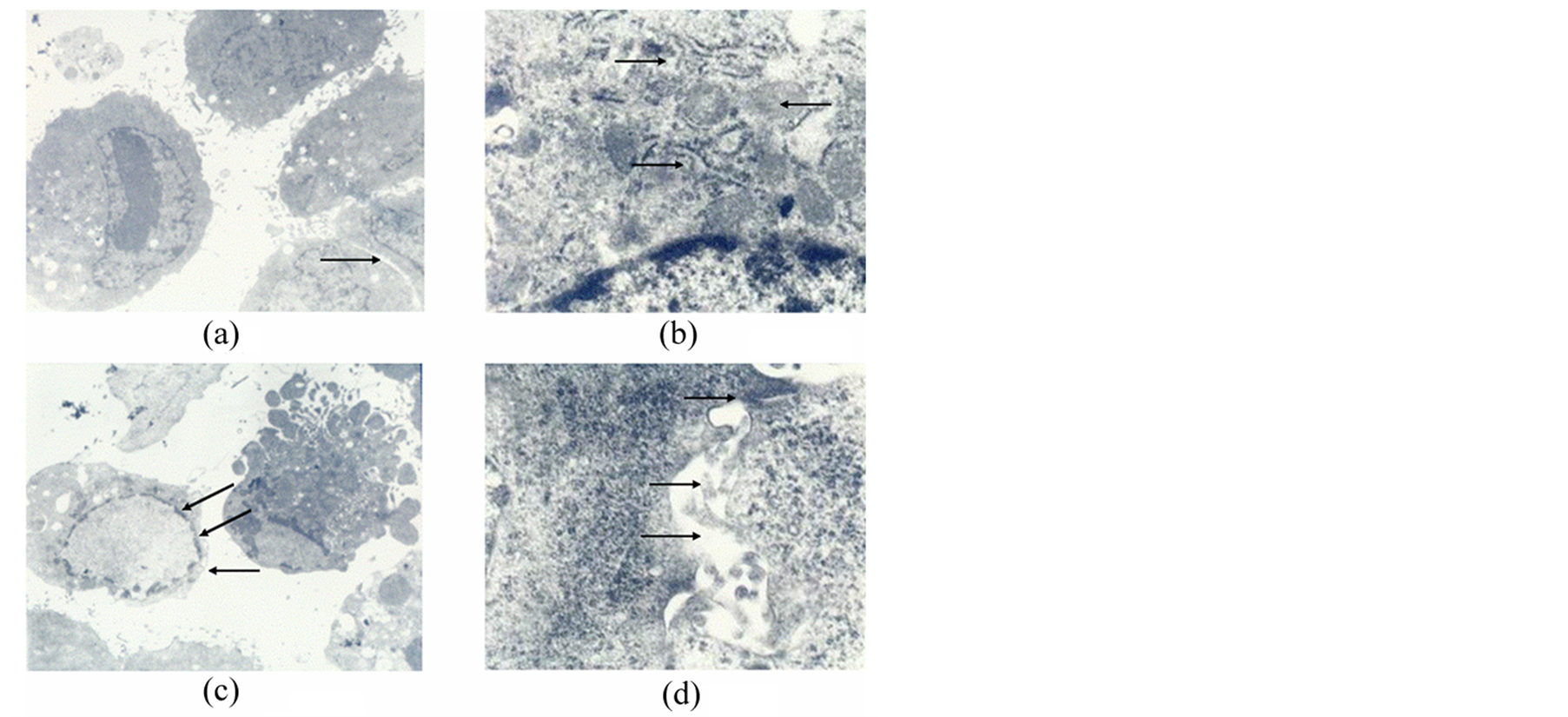
Figure 3. MSCs cultured in AS after RFA for 14 days. (a) Karyoplasmic ratio diminished. Original magnification 3500×; (b) Subcellular characteristics. Rough endoplasmic reticulum (up arrow), free ribosomes (down arrow) could be seen dispersed in the cell sap and mitochondria (middle arrow) increased .Original magnification 25,000×; (c) Typical apoptotic nuclear structures (arrows). Original magnification 3500×; (d) Cell-cell tight junction structures (up arrow), Slender microvilli (middle arrow) and cholangiole emerged (down arrow). Original magnification 30,000×.

Table 1. Cell cycle of of MSCs in different groups (Mean ± SD).
aP < 0.05 vs FCS and ASRF groups.
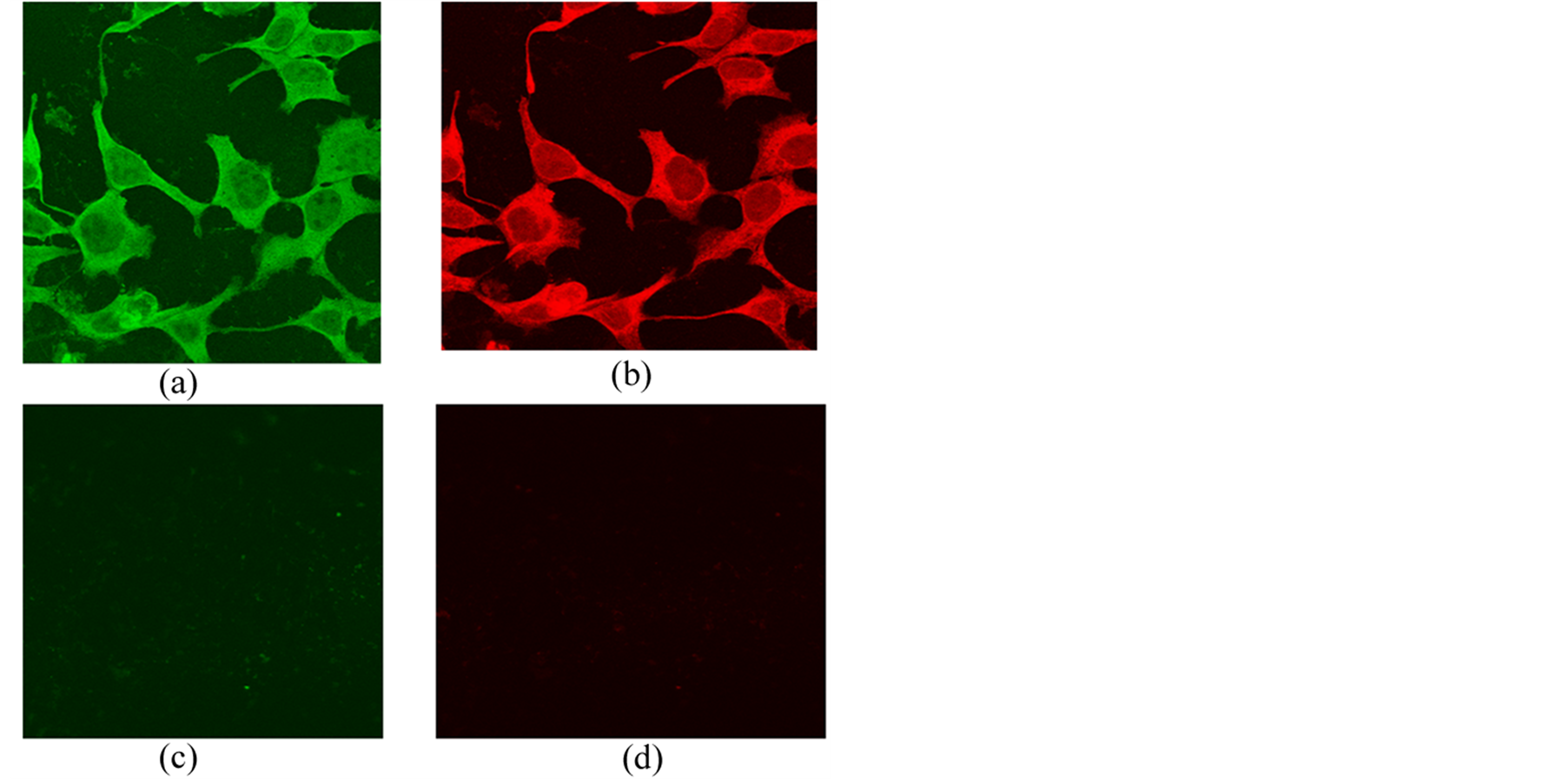
Figure 4. Immunofluorescence of MSCs cultured for 14 days. (a) Albumin expressed with FITC (green) and (b) CK18 expressed with TRITC (red) in ASRF group. Original magnification 400×; (c) No expression of FITC-labeled albumin and (d) No expression of TRITC-labeled CK18 in the FCS or AS group. Original magnification 400×.
in cultured MSCs from the FCS and AS groups (Figure 4(c) & Figure 4(d)).
4. Discussion
MSCs are multipotent cells that can be isolated from adult bone marrow and can be induced in vitro and in vivo to differentiate into a variety of mesenchymal tissues, including bone, cartilage, tendon, fat, muscle and liver [1] [14] . Accumulating evidence demonstrates the ability of bone MSCs to participate in liver regeneration after partial hepatectomy [15] . MSC-induced mobilization of CD34+ cells has been reported after liver resection in patients with primary liver carcinoma or metastasis [8] . Many growth and differentiation factors including HGF (hepatocyte growth factor), EGF (epidermal growth factor), TNF (tumor necrosis factor), and interleukin have been identified as being involved in this process [16] . For example, HGF has been identified as a mitogen for mature hepatocytes and is considered to be important for the development and regeneration of the liver [17] [18] .
Surgical resection still remains the most effective therapeutic approach for the treatment of hepatic tumors. Unfortunately, surgical operation is not feasible for some patients with marked cirrhosis and questionable liver function. RFA offers an alternative local treatment to un-resectable hepatic tumors and has recently been applied successfully in the field of surgical oncology [19] . Resection and RFA combined may play an even more important role in the management of HCC because of the high frequency of multifocal tumors and associated cirrhosis, which limit the applicability of extended resection to multifocal or bilobar HCCs [9] . Ablation of solid tumors with RF results from local resistive heating, which is produced when ions follow the oscillations of the highfrequency alternating electric field. A serious complication of RFA is the reduction of liver parenchymal volume, which may be critical for the metabolic demands of the organism. Whether bone MSCs are able to participate in liver regeneration after RFA of liver tumors is still an issue that needs to be addressed.
The expansion of MSCs in vitro has mainly been achieved in the presence of FCS, which might raise the risks of viral, bacterial and/or prion infection. Culturing MSCs in autologous serum may avoid these risks and retain the multi-lineage potential of MSCs. Thus, culturing MSCs in autologous serum for cell transplantion treatment is considered a promising strategy for the therapy of various liver diseases [3] . Whether bone MSCs can differentiate into hepatocytes in AS after RFA of a liver tumor remains unknown.
In our previous study, we investigated the optimal AS concentration for the expansion of rabbit MSCs [20] . A range of gradient concentrations from 1% to 70% of AS was applied, and the expansion rate to AS concentration exhibited a positive correlation within 50%. We chose the 30% AS after RFA therapy of the liver tumor as the suitable concentration for culturing MSCs into hepatocyte-like cells [21] . In the present study, we compared the differentiating effect of the medium supplemented with either FCS, AS or ASRF on cultured MSCs.
Fourteen days after culture, expanded MSCs in FCS and AS showed the general subcellular morphological characteristics of normal MSCs. In contrast, MSCs cultured in ASRF showed morphological characteristics similar to hepatocytes; free ribosomes and lysosomes, numerous lipid droplets, mitochondrial formation, slender microvilli, cell-cell junction structure and cholangiole were observed. These observations demonstrate a remarkable ability of the MSCs to differentiate in the ASRF group. Cell cycle analysis showed that the percentages of cultured MSCs in S/G2/M phase and the ratios of G1 to G2 in ASRF group are higher than those of the FCS and AS groups, indicating that AS after RFA was better for proliferation of MSCs. The higher percentage of apoptotic cells in the ASRF group may be due to some equilibrium mechanism of proliferation or defect something needed unclearly. Albumin is a typical marker of mature hepatocytes, whereas CK18 is expressed in several liver cell types including biliary epithelial cells and hepatic oval cells. The expression of albumin and CK18 was observed only in the differentiated hepatocyte-like cells in the ASRF group. However, the expression of these two markers is not associated with the expression of other hepatocyte markers and this expression does not necessarily demonstrate that these cells differentiated into true hepatocytes. For this purpose, more functional studies in animal models of liver disease are needed.
In conclusion, this study showed that MSCs can be expanded and induced into hepatocyte-like cells using AS after RFA in a rabbit model and this procedure may provide a potential cell source and culture process for clinical application in liver injury repair.
Acknowledgements
This study was supported “The Fundamental Research Funds for the Central Universities”.
NOTES
*Corresponding author.