Identification of N-Methyl Bis(2-(Alkyloxy-Alkylphosphoryloxy)Ethyl) Amines by LC-HRMS/MS ()
1. Introduction
An important content of the Chemical Weapons Convention (CWC) is the verification regime, which may include inspections of declared or suspected CW facilities. Samples are collected from these facilities and subjected to unequivocal identification of the Convention related chemicals (CRCs). For unequivocal identification of CRCs, the Organization for the Prohibition of Chemical Weapons (OPCW) maintains a network of designated laboratories by conducting official proficiency tests to evaluate their analytical capabilities [1] [2] . CRCs include not only the popular chemical warfare agents (e.g. Sarin and Soman) but also their precursors and degradation products. Consequently, a great deal of work has been devoted to build up MS database of CRCs and refine their analytical methods. In OPCW sample analyses, at least one of the data-rich spectrometric techniques (LC-MS/MS, GC-MS (EI), GC-MS/MS (CI), or NMR) is required to ensure unambiguous identification of CRCs. Comparison with reference data either from synthetic authentic chemicals or from database must be provided to make the identification.
N-Methyl bis(2-(alkyloxy-alkylphosphoryloxy)ethyl)amines (PNPs) are a series of newly designed and synthesized chemicals which belong to schedule 2.B.04 category of CWC. They are important markers of nerve agents and vesicant agents of N-mustards. Because of lack of mass spectra in the MS database, it is very difficult for the laboratories of Member States of OPCW to identify these new chemicals. Liquid chromatography coupled with high-resolution tandem mass spectrometry (LC-HRMS/MS) is one of the most favored analytical techniques for CWC related chemicals owing to its sensitivity, versatility, wide scan range, and high resolution [3] -[17] . LC-HRMS/MS was adapted to study the fragmentation behaviors of different N-Methyl bis(2-(alkyloxyalkylphosphoryloxy)ethyl)amines (PNPs). The analytical method of LC-HRMS/MS was developed and successfully applied to analyze one PNP chemical, N-Methyl bis(2-(butoxy-methylphosphoryloxy)ethyl)amine, in one organic sample of the 33rd official OPCW proficiency test.
The alkyl substitutes on phosphorus include methyl, ethyl, isopropyl and n-propyl as defined by CWC. The isoand n-propyl isomers must be unequivocally identified as required by the criteria stipulated by the Technical Secretariat of the OPCW. The O-alkyl substituents may contain 1 to 10 carbon atoms as defined by CWC. Therefore, a very large number of isomers are possible. But identification only in terms of molecular formulae is required. So differentiation of P-alkyl especially P-propyl substituents has become important in OPCW proficiency tests. We synthesized PNPs with all the four different P-alkyl substitutes. The O-alkyl substituents in PNPs of this paper contain 1 to 4 carbon atoms. The general structures of N-Methyl bis(2-(alkyloxy-alkylphosphoryloxy) ethyl)amines were shown in Figure 1. The detailed information of these chemicals was shown in Table1
2. Experimental
2.1. Materials
Methanol and water were of LC-MS grade, and all other chemicals used were of HPLC grade. N-Methyl bis(2- (alkyloxy-alkylphosphoryloxy)ethyl)amines were in-house synthesized. Purities were generally not less than 95%. They were dissolved in methanol (10 µg/ml).
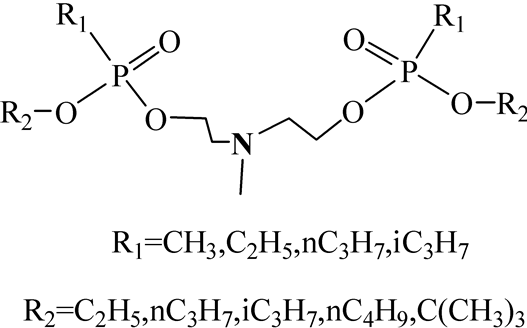
Figure 1. Structures of N-Methyl bis(2-alkoxy-(alkphosphoryloxy)ethyl)amine.

Table 1. Chemical names, abbreviations and structures.
2.2. LC Conditions
LC separations were performed using a Agilent 1200 Separation Module fitted with a 150 mm × 2.1 mm i.d. Zorbax Eclipse plus C18 (5 µm) packed fused-silica column. The mobile phase consisted of 0.05% formic acid in water (solvent A) and methanol (solvent B). The elution gradient was 95% A for 2 min, to 50% in 2 min, hold for 4 min, to 0% in 1 min, hold for 5 min, to 95% A in 2 min, hold for 4 min, at a flow rate of 0.25 ml/min. Injections of 1 μl were used for all analyses.
2.3. MS Conditions
MS analyses were performed using an Agilent 6520 quadruple-time-of-flight mass spectrometer fitted with an electrospray (ESI) interface, operated in positive ESI mode. Capillary voltage was set at 3.5 kV, capillary temperature at 350˚C, fragmentor voltage at 150 V. Evaporation gas was set at flow rate of 8 L/min and desolvation gas at pressure of 30 psig. ESI-MS data were acquired from 50 to 1000 Da with resolution of 10,000. MS-MS product ion spectra were collected from 20 to 1000 Da, Collision energy was set as 10 V.
3. Results and Discussion
3.1. LC Conditions
LC separation is very important for complex sample analyses because the background chemicals strongly influence the ionization efficiency in ion source. Retention times were relatively long because of the weak polarity of PNPs. Elaborate gradient elution condition should be needed if complete separation of PNPs is required. But the main purpose of this study is to build up LC-HR MS/MS database. So the general LC conditions when analyzing other CRCs were adapted.
3.2. Optimization of Collision Energy and MS/MS Analysis
LC-MS technique is a kind of soft ionization technique. Generally, the base ions are quasi-molecular ions or adduct ions, and there are only a limited number of fragment ions in mass spectra in LC-MS analysis. The elements composition of the chemicals can be acquired by LC-HRMS analysis, but it is difficult to determine the chemical structures. Using LC-HRMS/MS technique, chemical structures can be identified by analyzing the product ions of the abundant fragment ions in LC-HRMS. In this study, different collision energy was optimized and high quality MS/MS spectra were obtained on collision energy 10 V. TICs and LC-HRMS/MS spectra of different PNPs at the optimized condition were shown in Figure 2 and Figure 3. The related information including [M+H]+, RTs, mass errors and major product ions were given in Table2 The mass weights were accurate and the biggest mass error was 5.4 ppm.
3.3. LC-HRMS/MS Spectra Fragmentation Pathways of PNPs [18] [19]
These compounds showed different fragmentation patterns although their structures are very similar. The base ions were the quasi-molecular ion [M+H]+ in LC/MS and in MS/MS were the [M+H-CnH2n+1PO3]+ ions, which were presumably formed via loss of the alkyloxy alkylphosphoryloxy groups from the precursor ions. The diagnostic ion m/z84.0814 was identified as [C5H10N]+, which was the ion of (CH2=CH)2N+(H)CH3. O-n-propyl PNPs showed two fragmentation pathways. In one way, the quasi-molecular ion [M+H]+ lost a propoxy alkylphosphoryloxy group to produce [R1P(OH+)(O-n-C3H7)OCH2CH2N(CH3)CH=CH2]+, which could be fragmented further to produce [C5H10N]+ ion. In the other way, [R1P(OH+)(O-n-C3H7)OCH=CH2]+ ions were produced from [M+H]+ and fragmented further to produce the abundant ions [R1P(OH+)(OH)OCH=CH2], resulting from P-O cleavage with loss of alkene. O-isopropyl PNPs characteristically produced a weak fragment ion [M+ H-C3H6]+, which were presumably formed via loss of CH3CH=CH2 from [M+H]+. Other PNPs showed similar fragmentation pathways as O-n-propyl PNPs. The major fragmentation pathways were illustrated in Figure 4 and Figure 5.

Table 2. [M+H]+, RTs, mass errors and major fragment ions of chemicals.
It is easy to distinguish n-propyl isomer and iso-propyl isomer in real samples without the considerable time consuming in synthesizing a number of isomers. Take PNP-EPrX and PNP-EPX for example, under the same chromatographic conditions, the n-propyl isomer was eluted marginally faster than the isopropyl isomer. But, in the absence of pairs of standard chemicals, retention time was not a reliable indicator. Using ESI MS, van Baar et al., demonstrated that iso and n-propylphosphonic acids could be differentiated by the CID spectra of the protonated
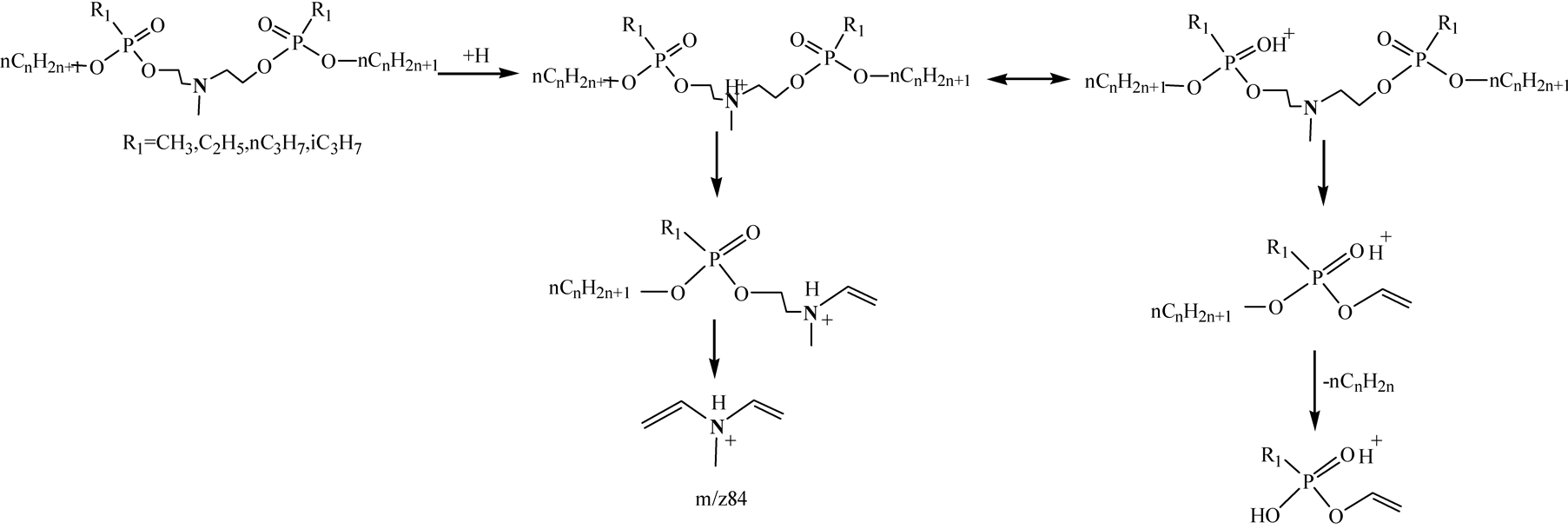
Figure 4. The major fragmentation pathways for N-Methyl bis(2-n-alkoxy-(alkphosphoryloxy)ethyl)amines.
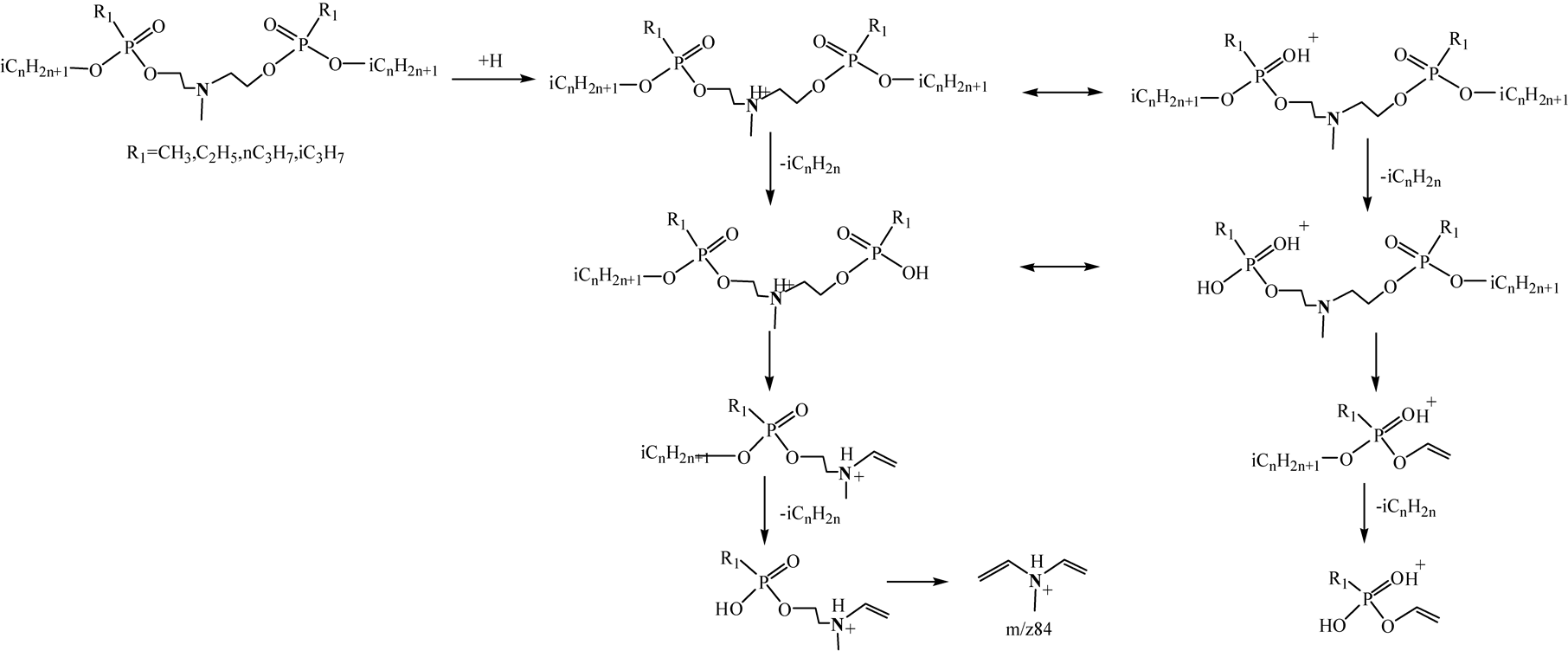
Figure 5. The major fragmentation pathways for N-Methyl bis(2-iso-alkoxy-(alkphosphoryloxy)ethyl)amines.
molecules [PrP(O)(OH)2+H]+. The CID spectrum of the protonated molecule of the P-isopropyl isomer PNP-EPrX showed a strong product ion, m/z 83 [MH-C3H6]+, resulting from P-C cleavage with loss of propene. Deuterium labeling experiments showed that it was involved a 1,4-hydrogen shift from one of the isopropyl methyl groups. However, this product ion was not observed with the P-n-propyl isomer. The isopropyl isomer also showed an intense product ion from further loss of H2O, m/z 65 [MH-C3H6-H2O]+, which was weak for the n-propyl isomer. In contrast, CID of the protonated molecule of the n-propyl isomer gave an abundant product ion from loss of water, resulting from P-O cleavage. But this ion was weak for the isopropyl isomer. We extended this methodology to study PNPs.
The LC-HRMS/MS spectra of the [M+H]+ ions of O-n-propyl and O-iso-propyl PNPs show a marked difference. The O-iso-propyl isomers showed relatively weak product ions as m/z346, m/z318 or m/z374 [MH-C3H6]+ from loss of propene, but these product ions were not observed with the O-n-propyl isomers. On the other hand, the O-iso-propyl isomers were easy to lose propene from O group on the other side during rearrangement (m/ z194, m/z208 or m/z180) after loss of alkylphosphonic acids.
3.4. Sample Preparation and Analysis for 33rd Official OPCW Proficiency Test
Our laboratory had assisted OPCW to prepare samples for 33rd official OPCW proficiency test. The newly designed and synthesized PNP-BX was deliberately spiked in an organic sample (OA). The background included diesel oil, tributyl phosphate and different stabilizers at very high concentration.
Because of spiking the new chemical, OA sample analysis was a challenge for Laboratories which took part in the 33rd official OPCW proficiency test. Only 5 Laboratories including sample preparation Laboratory and evaluation Laboratory out of 19 Laboratories identified PNP-BX correctly in 33rd official OPCW proficiency
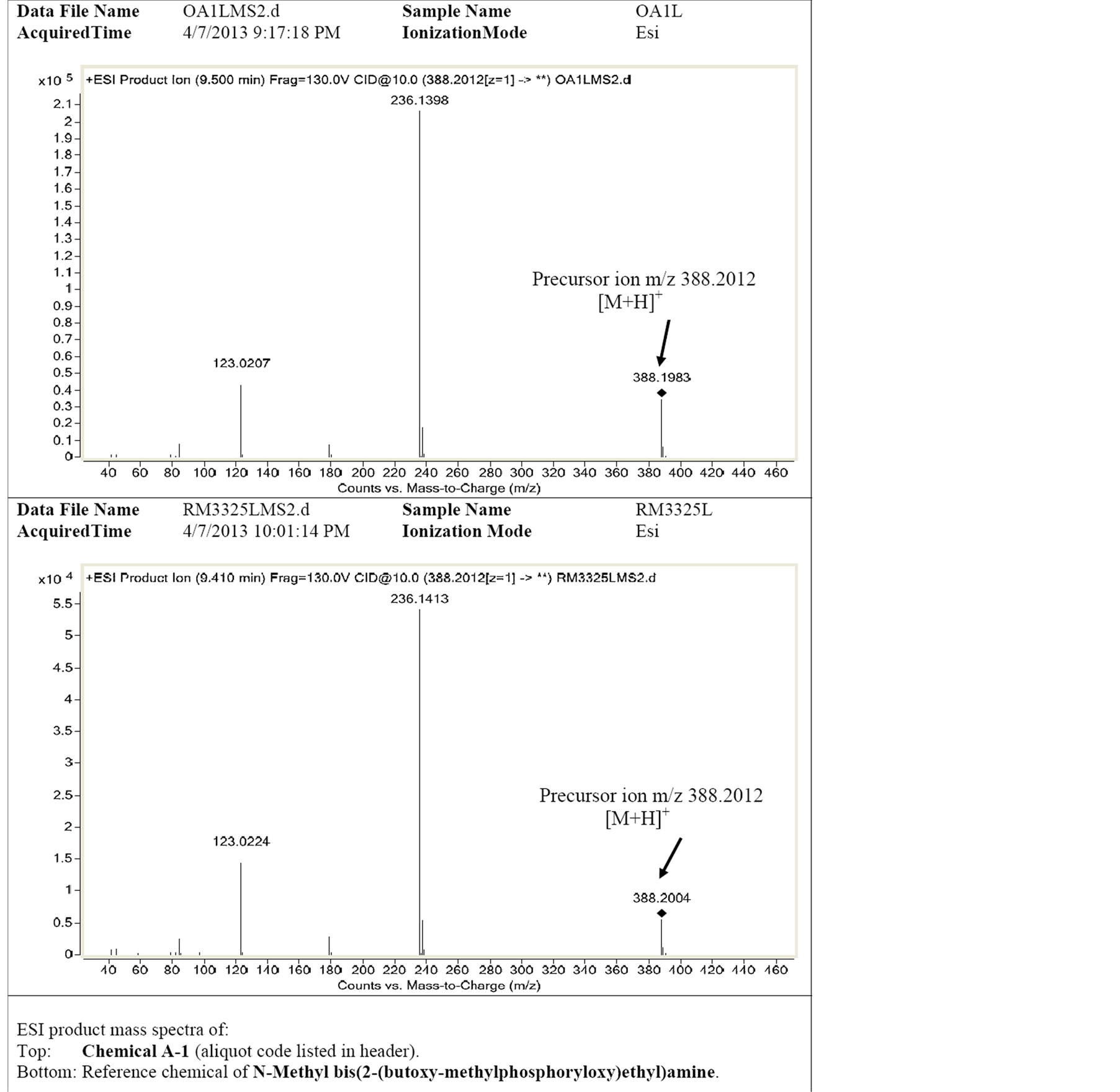
Figure 6. The LC-HRMS/MS spectra of N-Methyl bis(2-butyloxy-(methylphosphoryloxy)ethyl)amine in OA for 33rd official OPCW proficiency test (A) OA sample (B) reference chemical.
test. The LC-HRMS/MS spectra of PNP-BX in OA sample and of reference chemical were shown in Figure 6.
4. Conclusion
By LC-HRMS/MS technique, the characteristic fragmentation ions were analyzed for a series of new CWC scheduled chemicals, N-Methyl bis(2-(alkyloxy-alkylphosphoryloxy)ethyl)amines. The fragmentation pathways are different between O-n-propyl and O-iso-propyl isomers. The protonated molecules of the O-iso-propyl isomers produced relatively weak product ions from loss of CnH2n group, resulting from P-O cleavage. But the O-n-propyl isomers were not so. The LC-HRMS/MS database for CRCs including these PNPs has been built and it will be useful for unambiguous identification in environmental and biological samples for CWC inspections.