Nutritional Status of Patients with Cutaneous Leishmaniasis from a Tropical Area of Bolivia, and Implications for Zinc Bioavailability ()
1. Introduction
In tropical areas, especially in developing countries, nutritional deficiencies play an important role in the chronicity of some parasitic infections. Malnutrition is the most common cause of immunodeficiency worldwide; thus, epidemiological and clinical evidence suggests that nutritional deficiencies lead to an increased risk for development of infectious diseases due to impaired immunological responses [1].
Leishmaniasis is an infectious disease, endemic in 21 countries in America, and 39 million people in America are at risk for acquiring the disease [2]; it is a protozoan parasitic disease transmitted to humans by several phlebotomine sandflies. Depending on the strain of the parasite Leishmania, and the immunological status of the host, the infection may vary and develop from a skin ulcer (CL) to mucosal leishmaniasis (ML) leading to deformations, or to visceral leishmaniasis (VL) a severe form of systemic infection with hepatosplenomegaly [3]. Data about the prevalence of leishmaniasis in Chapare, a tropical area of Bolivia are scarce; most cases (85%) of CL are caused by the strain Leishmania (Viannia) braziliensis [4,5].
During the leishmaniasis infection, the impaired immune system of the host may cause an uncontrolled parasite replication that delays the healing of CL leading to a diffuse CL, ML or VL [6]. Even though it has been shown that leishmaniasis may occur in individuals in endemic areas independently of the nutritional status [7], studies with children have shown a link between poor nutritional status, growth retardation and iron deficiency with CL [8,9]. Furthermore, malnourished children are at a greater risk for developing severe VL than well-nourished children [10]. Malnutrition and micronutrient deficiencies are likely to interfere with several important functions of the immune system resulting in an impaired capability to overcome the leishmaniasis infection; nutritional status of the host is a key factor for the outcome of infection [10-12].
Nutritional status is evaluated primarily by dietary assessment methods that are widely used in both developed and developing countries for measuring the risks of nutrient deficiencies and excesses and evaluating the effects of nutrition interventions. Inadequate levels of nutrients originate either from primary deficiencies due to low levels of the nutrients in the diet or secondary deficiencies due to other factors like drugs, disease states or dietary components that inhibit the absorption of nutrients [13]. Secondly, anthropometric methods involving measurements of the body to provide an indirect evaluation of body composition are applied for the assessment of nutritional risks [13,14]. Furthermore, biochemical measurements of nutrients in biological fluids or tissues are used to detect subclinical deficiency states [13].
There is a lack of data on the nutritional status of rural populations in Bolivia, and no available data about nutritional status of patients with leishmaniasis. Recently we have studied the dietary patterns among a healthy population in Chapare; the results showed that their food consumption is mainly based on starchy tubers, cereals, and legumes providing 72E% from carbohydrates, with small portions of meat and eggs for protein 13E%, and with oil or tallow as a source of fat 15E% [15]. This type of diet has been associated with micronutrient deficits, notably iron, zinc, and calcium [16,17], due to the small amount of animal-source foods and the presence of mineral inhibitors like phytates, which is a strong chelator of minerals, reducing their bioavailability [18,19]. The inhibitory effect of phytates in mineral absorption appears to follow a dose dependent response, and the molar ratios Phy:Zn, Phy:Fe (phyate:iron) and Phy:Ca (phytate:calcium) in the diet have been used to predict the proportion of absorbable minerals [16,20,21].
Zinc and copper are essential trace elements of great importance for many enzymes and biological processes and their deficits or excesses may lead to different health problems [22]. Zinc deficiency in particular has a great impact on the defense mechanisms of the body and the immune response to infections. These have been well documented [23,24], but there is limited information about zinc and copper status and CL. Although a few studies have reported alterations in the status of these minerals during leishmaniasis [25,26], there has been no reported information about their relation to the dietary components.
This paper presents the dietary and anthropometric assessments of a group of patients with CL from the tropical area Chapare in Bolivia compared with healthy subjects. Additionally, in order to provide an estimate of the relative bioavailability of zinc, iron and calcium in the diet of the studied population, the content of phytate and minerals in their diet, were calculated and the molar ratios phytate: mineral are presented. Furthermore, biochemical indicators of zinc and copper were studied and correlated with the anthropometric and dietary features in order to contribute to the knowledge concerning the zinc and copper status in adult patients and gain some insight into the effect that phytates from the diet and the presence of the leishmaniasis infection may have on the absorption and metabolism of these minerals. The results of this nutritional evaluation are aimed to be used as a baseline in a further intervention study of zinc supplementation during leishmaniasis treatment.
2. Subjects and Methods
2.1. Study Participants and Design
The study was carried out in a tropical region located approximately 160 km east of Cochabamba, Bolivia, including the rural villages named Villa Tunari, Eterazama, San Gabriel, Aroma, Chimore, Shinaota and Ivirgarzama. Patients were recruited by contact with the local health centers; CL diagnosis was confirmed by microscopic examination of lesion smears and by isolation of parasites by culture according to the procedure previously described [27]; 34 patients were enrolled but complete data were collected for 32 patients.
The exclusion criteria for patients considered: patients with skin ulcers by another etiology (negative CL diagnosis), patients with previous leishmaniasis episodes, patients currently receiving leishmaniasis treatments or other drugs, patients with additional ML, patients with multiple CL lesions, patients taking mineral-vitamin supplements and pregnant or lactating women. In the aforementioned area 32 healthy control participants, of the same sex and approximately the same age (±5 years) as the patients, were enrolled. The exclusion criteria for healthy controls were: subjects presenting any disease at the moment, subjects with previous leishmaniasis episodes, subjects taking drugs or mineral-vitamin supplements and pregnant or lactating women.
All patients and controls signed a letter of consent prior to their participation. The study follows a casecontrol design, approved by the Ethics Committee of the Faculty of Medicine at Lund University and Faculty of Medicine at San Simón University.
2.2. Anthropometric Measurements
The anthropometric indicators, body mass index (BMI), mid-upper-arm muscle area (AMA), and mid-upper-arm fat area (AFA), were evaluated in patients and controls lightly dressed and without shoes. Measurements of weight were done with a digital electronic scale (Omron HBF400), 150 kg ± 0.1 kg, height with a portable staidometer ±1 mm, mid-upper-arm circumference in the left arm with a flexible non-stretch tape ±1 mm, and triceps-skin-fold in the left arm with a caliper ±0.2 mm (Harpender Skinfold Caliper, Baty International, United Kingdom). The indicators BMI, AMA and AFA were calculated with equations from the WHO committee [14], and evaluated according to the WHO and Frisancho classification [14,28].
2.3. Assessment of Dietary Intake
The dietary intake of the patients and controls was assessed during three consecutive days by Food Photography 24-hours Recall Method (FP24h-R) previously evaluated and described in detail [15]. Briefly explained the method is a 24-h recall supported by a photographic food record; subjects take digital photographs of all their meals and beverages consumed over a period of time, then nutritionists visit the subjects after each 24-h period to fill in a 24-h recall questionnaire with the detailed information of all the consumed foods. The portion sizes are estimated using the digital photographs taken by the subjects compared with standard food portions depicted in a photo atlas. Food consumption data were extracted from the questionnaires and the nutrient calculation was done in an excel file with a food data base from National Nutrient Data Base for standard reference [29] and a few items from the Bolivian Food Composition Table [30].
The calculations were performed for the intake of energy, protein, fat, carbohydrates, fiber, calcium, iron, phosphorus, zinc, copper, thiamin, riboflavin, niacin, pantothenic acid, folate, β-carotenoids, and vitamins A, C, E, B6, and B12. These nutrients were selected to elucidate differences in the nutrient intake between patients and controls and to shed light on possible deficiencies presented in this rural population; thus the subjects’ median daily dietary intake results were compared with the recommended nutrient intake (RNI) from World Health Organization (WHO) [31], according to sex and age of each patient and control. Additionally, data of the phytates content were included in the database according to a literature review [32-35]. The intake of phytates was calculated and the molar ratios Phy:Zn, Phy:Fe and Phy:Ca are presented to give some insight into the relative bioavailability of iron, zinc and calcium in the diet of patients and controls.
2.4. Trace Elements Indicators
After the diagnosis of CL was confirmed, blood samples (5 ml) were drawn from fasting patients and controls from the antecubital vein, into free trace element tubes; the samples were immediately centrifuged (5000 g at 4˚C for 10 min) in order to separate the serum, which was divided into aliquots and stored at −20˚C until zinc and copper analyses.
Serum zinc was quantified by flame atomic absorption spectrometry (Model 2280, Perkin Elmer Corporation, Norwalk, CT, USA), and serum copper by a graphite furnace atomic absorption spectrometry (Model SIMAA 6100, Perkin Elmer Corporation, Norwalk, CT, USA). Before analysis the samples were diluted 10 times with deionized water [36], and a calibration curve for each mineral was prepared from certified Atomic Absorption Standard solutions (Perkin Elmer Corp.). The reference material SeronormTM trace elements serum L-1-2 (SERO AS, Norway) was used to validate the mineral analysis.
2.5. Statistical Analysis
The normal distribution of the data was evaluated for all the parameters by Shapiro-Wilk test, and by measurements of skewness and kurtosis. Most of the parameters did not have normal distribution and thus all the results are presented as medians and percentiles 25th and 75th. First, the data were evaluated for continuous variables in the whole group of patients (n = 32) and controls (n = 32) using the statistic tests for matched data Wilcoxon rank test, and Chi-Square analysis was used to test the group differences in categorical variables, such as BMI, AMA and AFA classification. Later on, the groups were divided in female patients (n = 12) and controls (n = 12), male patients (n = 20) and controls (n = 20) and compared with Wilcoxon rank test to evaluate differences between same gender patients and controls.
Spearman’s correlations were computed to evaluate the association between the anthropometric indices (BMI, AMA, AFA) with energy and macronutrient intake, and between the biochemical measurements of zinc and copper with the corresponding intakes and with the anthropometric variables. Correlations between serum zinc with phytates and Phy:Zn were also calculated to elucidate the effect of phytates on the serum zinc. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 18.0 (SPSS Inc., IBM corporation 2010, www.spss.com). The significance level was set up at P values < 0.05.
3. Results
3.1. Anthropometric Measurements
Thirty-two patients and 32 controls participated in the study; the age range was between 14 and 50, and each group consisted of 12 females and 20 males. There were no significant differences in anthropometric results of BMI, AMA and AFA between patients and controls (Table 1). According to the WHO classification [14], most of the patients (50.0%) and controls (59.5%) were in the normal weight classification and there were no subjects in the underweight classification in any of the groups; hence 50.0% of the patients and 40.5% of the controls were overweight or obese and differences were not statistically different (P = 0.340). The AMA and AFA indicated that most of the patients and controls were in the average muscle and fat status according to the Frisancho classification [28] and not significantly different (P = 0.485 and 0.192 respectively). Wilcoxon rank test to compare groups of female patients (n = 12) with female controls (n = 12), and male patients (n = 20) with male controls (n = 20) showed no significant differences (results not shown).
3.2. Dietary Intake
Results of the dietary intake of patients and controls, were calculated as the nutrient density (nutrient/MJ) and
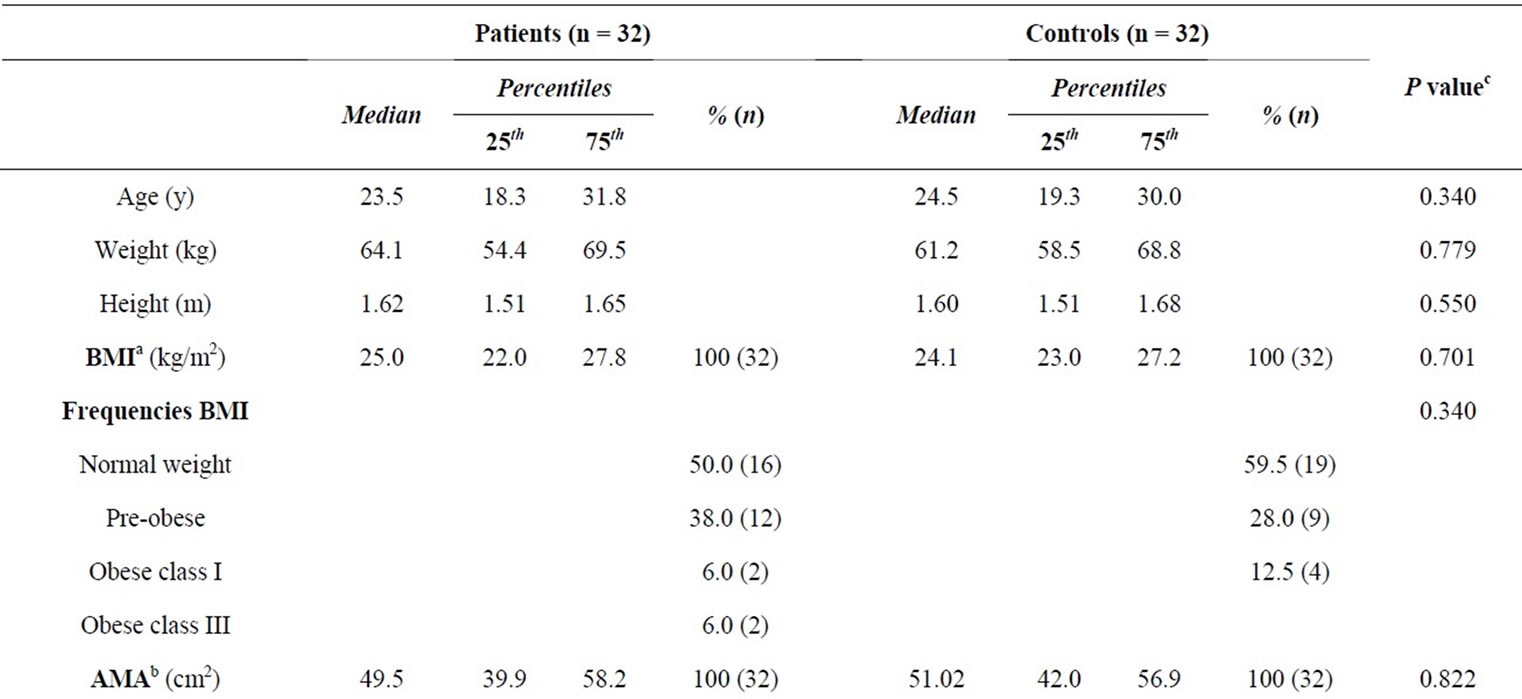
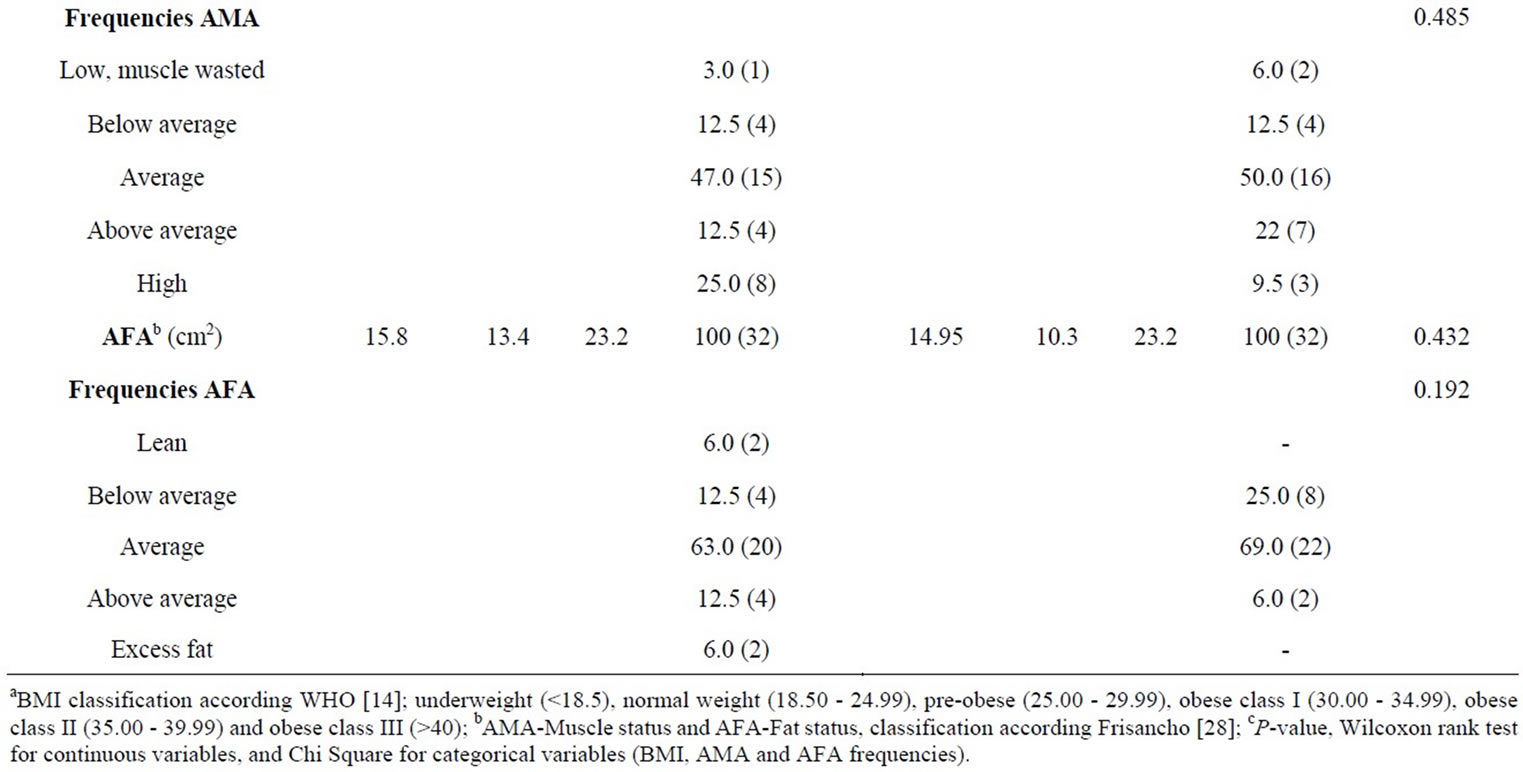
Table 1. Anthropometric characteristics of patients with cutaneous leishmaniasis and healthy controls.
compared by Wilcoxon rank test, which showed significant differences in fat and carbohydrates intake, but energy and most of the micronutrients were not statistically different (P > 0.05) between the two groups, excepting for that of Vitamin C (Table 2).
The median energy intake was generally low in both groups, consequently, the results were compared with their corresponding energy expenditure, calculated by equations from FAO/WHO [31]. The energy intake of patients met 78% and of controls 76% of their energy expenditure. The intake of calcium, folate, vitamin A and E, were low, patient’s intake met between 38% and 65% of the RNI and control’s intake met between 36 and 59 of RNI; the other nutrients showed values close to the recommendations (Table 2).
Besides, the median value of iron intake was lower in the group of female patients (12.5 mg/d) and female controls (12.6 mg/d), meeting only the 52% of RNI. Furthermore, in order to gain knowledge about food components affecting mineral absorption, phytates intake and molar ration phytate:mineral were evaluated (Table 2). Phy:Zn ratios were between 11 and 19 (25th - 75th), Phy: Fe was between 7 and 9, and Phy:Ca between 0.14 and 0.35.
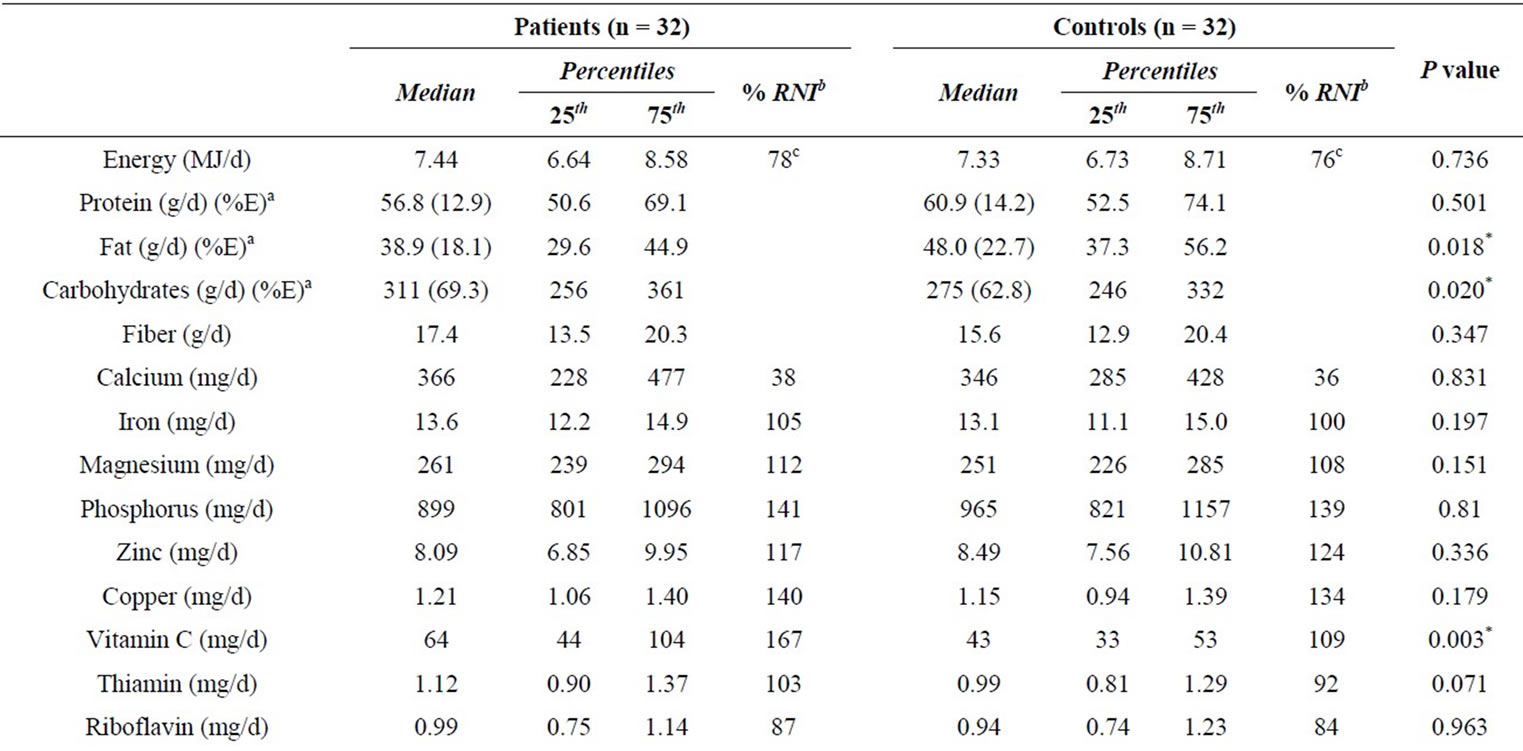

Table 2. Nutrient intake of patients with cutaneous leishmaniasis and healthy control group.
3.3. Trace Elements Indicators
Serum zinc in patients (80 μg/dl) was significantly lower (P = 0.033) than in controls (85 μg/dl) and serum copper was not significantly different (Table 3). The results of serum zinc were compared with reference values from NHANES III (90 µg/dl for females and 98 µg/dl for males and lower cut-off 70 and 74 µg/dl for females and males respectively) [38]. The median values of serum zinc were below the average reference values, in 88% of the patients and 79% of the controls, furthermore, 29% of patients and 15% of controls showed zinc serum levels below the lower cut-off, indicating that they are at risk of zinc deficiency. Values of serum copper were within the range of reference values (70 to 140 µg/dl) [39].
Correlations between anthropometric indicators BMI, AMA, AFA and intake of energy, protein, fat, and carbohydrates are presented for the groups of all patients, all controls, male patients, male controls, female patients and female controls (Table 4). BMI was positively correlated (P < 0.001) with the muscle and fat status for all groups; the correlations were stronger for females patients and controls (P < 0.001) than for males patients

Table 3. Zinc and copper serum levels of patients with cutaneous leishmaniasis and controls.

Table 4. Correlations of BMI, muscle and fat status with energy and macronutrients intake.
and controls (P < 0.05). The associations between BMI and energy intake were positive for all groups, significantly so for male and female patients but not for male and females controls. A similar tendency is shown for correlation between BMI with protein and fat. AMA was positively related to protein and fat intake for male controls (r = 0.555 and 0.569, P < 0.05) but not significantly for male patients not for females.
There were no significant correlations between AFA with energy and macronutrient intake for any of the groups. Also no significant correlations with serum zinc and copper for any of the anthropometric variables for either group were found (results not shown).
Serum zinc was correlated with zinc intake at level P < 0.05 for the groups of all patients and female patients (Table 5). Further, serum zinc presented a negative significant correlation (P < 0.05) with copper intake for the groups of female controls (r = −0.657). Negative correlation with phytates intake was found for all groups; significant (P < 0.05) for the group of male patients (r = −0.488), male controls (r = −0.460). In addition, the molar ratio Phy:Zn for daily intake showed negative correlations (P < 0.05) for all groups except for male patients. Serum copper was negatively correlated with zinc intake for all the groups, significantly (P < 0.05) for all patients (r = −0.361). The correlations between serum copper and copper intake were not completely conclusive; some of them were negative and others positive.
4. Discussion
One of the most interesting findings of the present study is the significantly lower serum zinc concentrations found in patients with CL compared with the healthy controls; this might be associated with the presence of leishmaniasis. Another interesting finding was the apparently lower serum zinc status of both patients and controls in spite of a zinc intake according to the recommended values. This was most likely due to zinc absorption being impaired for phytates content in their diet.
The zinc dietary intake of patients and controls met the dietary recommendations (7.8 mg/d for females and 7.0 mg/d for males) [40], however, the zinc in serum of both groups was below the average reference value (90 - 98 μg/dl) [38]. Furthermore, the serum zinc in patients was significantly lower than in healthy controls. The results are consistent with previous studies [25,26,41] where serum zinc was significantly lower in CL patients.
Diminished serum zinc was also seen in patients with ML [41] and, to a greater extent, in patients with VL [41,42]. The decreased serum zinc levels in patients with leishmaniasis are probably due to the redistribution of zinc from plasma to the liver. Cytokines (IL-1) released during the acute-phase response of the host’s immune system activates the synthesis of metallothionein in the liver and other tissues; metallothionein participates in the process of energy production and protection against reactive oxygen species that may be generated during the

Table 5. Correlations of zinc and cooper serum levels with the corresponding dietary intakes.
infection, and it is a metal-binding protein which appears to alter the hepatic uptake of zinc [43]. A study with mice has demonstrated that, besides metallothionein, the zinc transporter Zip14 contributes towards the reduction of the zinc levels during inflammation and infection [44].
In recent years, there has been a great interest in the study of the zinc status and supplementation, since the demonstration of its critical role in reducing the risk and severity of diverse infections [21], and of CL in particular, because it is known that wound healing is impaired in zinc deficiency [1], and that oral zinc administration in the treatment of acute CL has shown a good response in the healing of the lesions caused by the infection [45].
Besides the changes of serum zinc during leishmaniasis, it has been reported that levels of serum copper were higher in patients with CL [25,26,41,46], ML and VL [41]. The increased level of serum copper could be attributed to inflammation due to the presence of the leishmaniasis parasite [25]. In the present study the levels of serum copper were not significantly different between patients and controls. Further studies are needed in order to investigate the changes of copper during the course of the infection, and the use of more sensitive indicators to detect changes in copper status [47].
Complex mechanisms might be involved in bringing about the differences in serum zinc and copper in patients with CL and control subjects, most of them produced as a consequence of the acute-phase response of the defense strategies of the host’s immune system as mentioned above. Immune cells, just as any other cells, require an adequate supply of trace elements, so there is a redistribution of essential minerals like zinc and copper and an increase in the hepatic synthesis of acute-phase proteins like ceruloplasmin [48]. Another enzyme involved in the immune response is superoxide dismutase (SOD) which requires both zinc and copper for its normal activity; copper is necessary for catalysis and zinc stabilizes the enzyme. In this sense there is a competition between the two minerals to reach the enzyme, which may cause the imbalance of the minerals [49].
The low levels of serum zinc were not only found in the patients with CL but also in healthy controls with an adequate zinc intake according to the RNI from WHO/ FAO [31]. The zinc intake of patients and controls met the zinc requirements in 117% and 124% respectively. However, 88% of the patients showed values below the reference serum zinc value and 29% of them presented values below the lower cut-off, indicating that they were at risk for zinc deficiency. In the healthy controls, 79% of them presented values below the reference and 15% of them were at risk for zinc deficiency. These results drew attention to the need to investigate other factors besides the leishmaniasis that could decrease the levels of serum zinc in both patients and healthy controls.
Among the causes of the low serum zinc concentrations are; a low dietary zinc intake and a low absorption of dietary zinc as a result of other components in the food and the physicochemical interactions in the intestine; low serum zinc levels may often be due to a combination of these factors [13,22,38]. In the present study the zinc intake was not the cause of the low serum zinc, so it was most likely, due to the presence of zinc inhibitors in the diet, such as phytates, which are strong chelators of divalent minerals and are primarily to be found in cereal grain, legumes, seeds and tubers [17,22], which are the principal components of the diet in this area as we have previously shown [15].
The diet of the studied population showed Phy:Zn between 11 and 19 (25th -75th), indicating that zinc absorption may be compromised for the level of phytates content. According to the WHO committee [40] Phy:Zn higher than 15 are considered to inhibit zinc absorption and even molar ratios between 5 and 15 may have a certain negative effect on the absorption of zinc. It was reported that diets in rural areas following similar dietary patterns with high Phy:Zn impair mineral bioavailability, leading to zinc deficiencies [16,17,50-52]. Besides, the Phy:Fe (7 to 9) was much higher than 1, which is the level considered adequate for iron bioavailability [53]. Furthermore, Phy:Ca (0.14 to 0.35) was higher than the desirable value 0.17 [54], indicating that it is also likely that phytates compromised iron and calcium absorption in this diet.
The association between serum zinc and dietary zinc, phytates, and Phy:Zn (Table 5) showed positive correlations (P < 0.05) between serum zinc and zinc intake, indicating that serum zinc is a good indicator to reflect dietary zinc, as has been demonstrated in other studies [55,56]. Correlations between serum zinc and phytates intake were inverse and significant (P < 0.05) for male patients (r = −0.488) and male controls (r = −0.460). Stronger significant inverse correlations (P < 0.05) were found between serum zinc and Phy:Zn for all the groups (except for male patients), indicating that the zinc absorption was impaired by the presence of phytates in the diet. In a study of vegetarian and omnivorous diets, a similar inverse significant association was reported between serum zinc and Phy:Zn in women with low levels of serum zinc [57]; the same findings were reported in a study with women from New Zealand [58]. Associations between serum copper and the corresponding copper intake were not conclusive; most probably because this biomarker is not sensitive enough to reflect copper intake, except when a severe deficiency is present or the intake is very low [22,59].
In relation to the anthropometric characteristics, there were no significant differences between the median values of BMI, AMA and AFA between patients and controls (Table 1). None of the patients or controls was underweight. However, 50.0% of patients and 40.5% of controls were overweight or obese and similar results were presented by Ferreira da Cunha et al. [60]. Overweight and obesity is not an indication that the patients have a better nutritional status; they may have micronutrient deficiencies and an impaired immunity associated with the consumption of imbalanced diets [61,62]. The increased prevalence of overweight is a consequence of the dietary transition reported in Latin America with a reduced consumption of fruits and vegetables and an increase in fats and sugar [63].
The analysis of the dietary intake showed that the basic diet of patients and controls present a composition of macronutrients as energy percentage, within the dietary recommendations from WHO [31]; 69.3 and 62.8 E% from carbohydrates, 12.9 and 14.2 E% from protein and 18.1 and 22.7 E% from total fat for patients and controls respectively. These results are consistent with those reported in a study of dietary intake carried out in the same area, where the contribution from carbohydrates was 72E%, proteins 13E%, and total fat 15E% [15]. The energy distribution is, in accordance with the dietary pat terns of the area, based primarily on carbohydrates from cereals, tubers and legumes, protein from small portions of meat or eggs and fat from oil and tallow; the vegetables and fruits are present in small portions. The diet of the subjects varied very little from one day to another constituting a monotonous diet. The median energy intake was, in general, low: 7.44 MJ/d for patients and 7.33 MJ/d for controls. Similar dietary and energy intake patterns were found in a rural population in Argentina, where energy intake was between 6.65 to 7.77 MJ/d [64].
Furthermore, the nutrient intake of patients and controls in this studied population indicates the existence of deficits of several essential nutrients; calcium, iron (for women), folate, Vitamin A, and E, with median values below the 65% of the RNI. Micronutrient deficiencies have been seen among rural populations, especially in rural areas in developing countries with dietary patterns containing small amounts of animal foods, which may lead to a micronutrient malnutrition or “hidden hunger” [65,66].
The results in this study indicate that the infection of CL does not stand in direct association with the nutritional status shown by the anthropometric and dietary assessments. Thus, the infection may randomly affect the exposed individuals, independently of their current nutritional status. However, further studies are needed in order to determine whether the development of the disease is exacerbated by a low nutritional status. There are few studies about the association of CL and nutritional status; in children a more severe clinical manifestation of leishmaniasis was found when chronic energy-protein malnutrition was present [9], and it was also reported that in patients older than 22 years of age the risk of severe manifestations of leishmaniasis increases when the nutritional status decreases [67]. Studies of the nutritional status and the outcome of VL have shown that malnourished children presented a more aggravated state of the infection, creating a circle of malnutrition and infection, causing low growth-rate and nutritional deficiencies [7,10].
5. Conclusions
The present paper shows that the serum zinc levels of patients with CL were significantly lower than those of healthy subjects. Furthermore, it was found that even though the zinc intake of patients and controls met the dietary recommendations, the serum zinc levels were below the average reference values, indicating a low absorption of dietary zinc in both healthy subjects and patients with CL. Indeed, the results showed that zinc absorption and metabolism might be compromised by inhibitory components in the diet, such as phytates, and by the presence of the CL infection. Additionally, CL was found to not be directly associated with the nutritional status observed in the anthropometric and dietary results. However, the results of dietary patterns and nutrient intake shed some light on the existence of deficits of several essential micronutrients, which are below the recommended intake.
Studies of the nutritional status of the population at risk for acquiring leishmaniasis are of great importance for the design and implementation of new strategies, both nutritional and therapeutic. In order to prevent complications in the outcome of leishmaniasis, as well as other adverse effects of imbalanced diets and nutritional deficiencies among rural populations in developing countries, the nutritional status of the host should be appropriately considered.
6. Acknowledgements
We thank Miguel Guzmán of Biomedical Research Institute, San Simon University, Cochabamba, Bolivia, for the collaboration providing blood samples. Financial support from the Swedish International Development Agency (SIDA/SAREC) is gratefully acknowledged.
NOTES