Volatile Organic Compounds in Crude Coconut and Petroleum Oils in Nigeria ()
1. Introduction
The potential use of coconut oil as a biofuel has prompted significant economic and environmental research in recent years. Biofuel is produced by transesterification of oil by reacting it with methanol in the presence of a catalyst such as NaOH to form methyl esters as biofuel and glycerin as a by-product [1] [2] . Burning of coconut oil releases less CO2 into the atmosphere as compared to petroleum oil [3] . Coconut oil is also a renewable resource. Even though the specific energy, viscosity, and cetane number are similar in coconut and petroleum oils, coconut oil has comparatively more physiochemical limitations related to relatively high viscosity, iodine concentrations, boiling point, and cloud point/ wax formation as well as the presence of residual water [4] [5] [6] [7] [8] . Nevertheless, coconut oil has the most suitable physical properties among all vegetable oils for biofuel generation. Table 1 compares some physical properties of coconut and petroleum oils. The extensive use of coconut in the production of biofuel may affect the diversity of the agroecosystem by shifting the farming interest to coconut potentially compromising the production of other crops and affecting the price of coconut [9] .
Crude petroleum oil is a complex liquid hydrocarbon that occurs naturally, consisting hundreds of hydrocarbons with some sulfur, oxygen and nitrogen impurities. Its combustion contributes greatly to historically increasing atmospheric carbon dioxide (CO2) concentrations, a factor in climate change. Some petrochemical oil combustion products are harmful to human [10] [11] [12] [13] . Leakage and spillage of petroleum oil during transportation can adversely affect the marine environment [14] .
Volatile organic compounds (VOCs) vaporize easily at ambient temperatures. Tucker defined VOCs as a class of carbon-containing chemicals that participate in photochemical reactions in ambient air [15] . VOCs are also known to have boiling points of ≤100˚C and/or vapor pressures >1 mmHg at 25˚C [16] . VOCs are found in many products such as fuels, solvents, paints, adhesives, and refrigerants, and are abundant automobile engine exhaust [17] - [22] . Indeed, the presence of VOCs in the environment continues to be a major subject of public concern and active research. In light of the great impact of VOCs on human health and environment, the objective of this paper is to examine the types and concentrations of VOCs in crude coconut oil and compare them to those in crude petroleum oil. This will help characterize the chemical signature of crude coconut oil for potential forensics use or in biofuel research.
2. Experimental Procedures
2.1. Samples and Reagents
Crude coconut and petroleum oils were obtained from Department of Petroleum Engineering, University of Ibadan, Nigeria. These oils were collected around the city of Ibadan, Nigeria. A sample (20 g) of each oil was placed in individual headspace vials, which were subsequently sealed. NIST-traceable and certified
![]()
Table 1. Some physical properties of coconut and petroleum oils.
standards were used to calibrate the GC/MS. All purchased reagents were analytical grade and used without further purification. Pure standards were purchased from Sigma-Aldrich (USA). Stock standard solutions for each of the analytes were prepared in a 100 mg/100 ml of methanol. Ultra-high-purity grade helium was obtained from Air Liquide (USA). Helium was used as the carrier gas in the GC runs. Ultra-high-purity grade nitrogen was produced in the laboratory using nitrogen generator for use as make-up gas. Compressed air and hydrogen were purchased from Air Liquide.
2.2. Gas Chromatography
Sealed vials containing the oil samples were placed in a thermostat to drive the volatile components into the headspace for analysis. An aliquot of the vapor phase was introduced through a gas-tight syringe or the sample loop of a gas sampling valve into a GC. The GC was configured for: 500 µL split/splitless injection at 200˚C, an initial oven temperature of 40˚C for 5 min, a final temperature ramp from 40˚C to 235˚C at 5˚C∙min−1, and a helium carrier gas velocity 1.25 mL/min. The MS was configured for a 0 min solvent delay, temperature of 280˚C, 2200 EMV, and 45 to 450 MS scan at 1.9 Scan/s. Headspace-GC cycle time was set at 60 min, and the injection time was set 1 min. The oven temperature was held at 95˚C, the Tr line temperature was 150˚C, and the loop temperature was 100˚C. The loop EQ time was 0.05 min, the loop fill time was 0.20 min, the pressure time was 0.20 min, and the vial EQ time was 15.0 min.
Quantitative analysis of samples was performed using an Agilent 7890A gas chromatography equipped with a flame ionization detector and a DB5 capillary column (25 m × 0.25 mm). Internal standards were used to calculate the concentrations of VOCs by the following equation [23] :
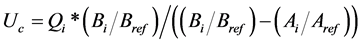
where i and ref represent a VOC to be determined and the internal standard, respectively. Standards and actual samples were run using the same experimental conditions, and VOC retention times were compared. Internal standards are carefully selected so that their peaks are close to but completely separate from the peaks of i. Bi/Bref is the ratio of the peak areas of i to the internal standard after being added to the sample being analyzed. Ai/Aref is the ratio of the peak areas of i to the internal standard before it is added. Qi is defined as the amount of internal standard added in µg, and Uc is the calculated amount of the VOC in µg. The relative response factors and recovery factors of n-alkane internal standards were also calculated. The relative response factors and recovery factors of the investigated VOCs were determined based on the internal standard with similar structures. Finally, concentrations of VOCs were then adjusted for their recoveries.
VOCs were identified by mass chromatography, using Agilent 780A gas chromatograph equipped with a mass spectrometer. An attached auto sampler was used for the qualitative identification of VOCs. The GC used a 25 m × 0.25 mm i.d. × 0.25 µm film thickness HP5 fused silica capillary column and liquid auto sampler. GC conditions were 1 mL split/splitless injection (injector temperature of 270˚C) at 60˚C, a splitless time of 60 s, a 3 min hold, a temperature increase of 3˚C∙min−1 to 300˚C, and a helium carrier gas velocity 30 cm∙s−1.
A minimum detection limit (MDL) of less than 500 parts per trillion (ppt) was determined. Five calibration points were used to establish linearity for the quantitative analysis of target compounds. The calibration was verified for each compound using the calculated Relative Response Percentage and the Correlation Coefficients value for linearity. Before any analysis was completed, a certification blank was run through the column to establish a baseline. During calibration and sample analysis, a blank was inserted between each sample to ensure that no carry-over or contamination occurred.
3. Results
A representative chromatogram is shown in Figure 1 for crude coconut oil. Mass spectra of the most abundant VOC in coconut oil (dodecanoic acid) and petroleum oil (toluene) are shown in Figure 2. The data are referenced to the mass spectral databases of US-NIST/EPA/NIH mass spectral library-NIST98.
VOCs found in the crude petroleum oil samples are listed in Table 2. Alkane compounds identified in crude petroleum oil were 2-methylbicyclo[3.2.1] octane, 5-ethyl-2,2,3-trimethyl heptane, decane, dodecane, octane, pentadecane, tetradecane, and tridecane. Among the eight alkanes found in crude petroleum oil, tetradecane had the highest concentration at 10.39 ppb and octane had the lowest concentration at 0.27 ppb [24] . Four alkene compounds in crude petroleum oil were identified: 2-methyl-2,4-hexadiene, 2,6-octadiene, butadiene, and octadiene, the latter of which had the highest concentration (5.68 ppb). Two alkyne compounds, 3-heptyne and octyne, were found in crude petroleum oil. Remarkably, no alkane, alkene, or alkyne compounds were found in crude coconut oil. VOCs found in the crude coconut oil samples are listed in Table 3.
Four aromatic compounds were found in crude petroleum oil including benzene, naphthalene, toluene, and xylene. Toluene was the most abundant VOC in
![]()
Figure 1. A representative gas chromatogram of crude coconut oil.
![]() (a)
(a) ![]() (b)
(b)
Figure 2. Mass spectra of the most abundant VOC of a) dodecanoic acid in crude coconut oil and b) toluene in crude petroleum oil.
crude petroleum oil at a concentration of 12.61 ppb; the mass spectrum of toluene is shown in Figure 2(b). Naphthalene was found at a concentration of 11.83 ppb in crude petroleum oil but was not present in crude coconut oil. Similar concentrations of benzene were found in crude coconut oil (2.68 ppb) and crude petroleum oil (3.68 ppb). Benzofuran was found in crude coconut oil (1.38 ppb) but was not detected in crude petroleum oil.
Two halogenated compounds (1-bromocyclooctene and dichloromethane) were found at low concentrations in crude petroleum oil, but none were detected in crude coconut oil. Three nitrogenated compounds, imidazole, indene-3- ethanamine, and s-triazolo[4,3-a]pyridine, were only identified in crude petroleum oil. Conversely, butanamide and phenylserine were only found in crude coconut oil. One sulfurous compound, 2-ethylhexyl tetra-sulforous acid, was found in crude petroleum oil at a very small concentration (0.20 ppb) but was not detected in coconut oil. Crude petroleum oil was found to have five oxygenated compounds, whereas crude coconut oil found to have four compounds. Cyclopentanol was the only one that was present in both crude oils. Hydromycrene, non-6-en-2-one, and thujone were found in crude petroleum oil. Dodecanoic acid was identified as the most abundant VOC in crude coconut oil (Figure 2(a)); carbamic acid and oxindole are other oxygenated compounds found in crude coconut oil.
Figure 3 shows the percentage of compounds by classification. For crude coconut oil (Figure 3(a)), oxygenated compounds account for 75% of the total VOCs, followed by aromatic compounds (20%), and nitrogenated compounds (5%). Figure 3(b) indicates that aromatic compounds comprise of 34% of the total VOCs in crude petroleum oil, followed by alkanes (32%), alkynes (11%), oxygenated compounds (10%), alkenes (8%), nitrogenated compounds (4%), and halogenated compounds (1%). The data from Figure 3 suggest that the
![]()
Table 2. Concentrations (ppb) reported for VOCs found in crude petroleum oil*.
*Each value is the average of three runs.
![]()
Table 3. Concentrations (ppb) reported for VOCs found in crude coconut oil*.
*Each value is the average of three runs.
![]() (a) (b)
(a) (b)
Figure 3. Comparison of VOCs measured by ppb found in crude coconut and petroleum oils. (a) Crude coconut oil; (b) Crude petroleum oil.
amount of nitrogenated-based VOCs in both oils are about the same level, whereas the level of oxygenated compounds in coconut oil is 7.5 times more than petroleum oil, though both contain different compounds. Total VOC concentrations were calculated as 87.46 and 20.49 ppb for crude petroleum oil and crude coconut oil, respectively.
4. Conclusion
Different VOCs were found in crude coconut and petroleum oils sourced from Ibadan, Nigeria. Twenty-nine VOCs were identified in crude petroleum oils, spanning eight chemical groups. Aromatic compounds and alkanes made up 66% of the total. Eight VOCs were identified in crude coconut oil; four oxygenated compounds accounted for 75% of the total VOCs. Remarkably, crude coconut oil contains no alkanes, alkenes, and alkynes. The total VOCs concentration of crude petroleum oil is about 4.3 times higher than crude coconut oil, suggesting that coconut oil is far more environmental-friendly than petroleum oil. Results obtained from this study reveal the types and amounts of VOCs found in crude coconut oil and petroleum oils, which serve as a framework for chemical signature for future forensics use or biofuel study.
Acknowledgements
Authors would like to thank H. L. Adebowale and E. O. Oyedokun of University of Ibadan, Nigeria for samples and ANA-Lab, Kilgore, TX for gas chromatography analysis.