Ring-Chain Tautomerism of the Condensation Products of 2-Mercaptobenzohydrazide with Aliphatic and Aromatic Aldehydes: Influence of Electronic and Steric Factors ()

1. Introduction
The tendency of functionally-substituted hydrazones to undergo intramolecular cyclization at the polar C=N bond of the hydrazone fragment is commonly used in the synthesis of five- and six-membered heterocycles [1] [2] [3] . This reaction is sometimes reversible, which leads to the coexistence of the hydrazone and cyclic forms as their tautomeric mixture in solution [4] [5] [6] . Thus, the 2- mercaptobenzoylhydrazone of acetone transforms in solution to the alternative 1,3,4-benzothiadiazepine form [7] . We note that the hydrazones obtained using the hydrazides of 2-hydroxy- and 2-aminobenzoic acids have linear structure. The possible cyclization at the C=N bond by attack of the available OH or NH functions does not occur [8] [9] .
The aim of the present work, being a continuation of the previous investigations, was to study the structure of the products of condensation of 2-mercap- tobenzoic acid hydrazide (2-HSC6H4CONHNH2) 1 with a series of aliphatic and aromatic aldehydes, and also the effect of the steric or electronic properties of a substituent in the aldehyde component on the position of the tautomeric equilibrium (Figure 1 and Figure 2).
2. Results and Discussion
Compounds 2a-2h and 3a-3h were obtained in 55% - 90% yield after briefly maintaining equimolar quantities of 2-mercaptobenzoic acid hydrazide 1 and the appropriate aldehyde during 10 - 15 min in methanol solution at room temperature (Experimental part). In all experiments, the 1H- and 13C-NMR spectra were recorded at definite time intervals starting from the moment of dissolution until the end of transformations. In addition, the structure of the compounds under study in the crystalline state was confirmed by solid-phase high-resolution 13C-NMR spectroscopy (CPMAS).
The variation in time of the 1H-NMR spectra of compounds 2a-2h suggests that they exist in the crystalline state in the cyclic benzo-1,3,4-thiadiazepine form B. For instance, in the freshly prepared solution of compound 2a in DMSOd6 a single set of peaks is observed corresponding just to B form in 1H-
![]()
Figure 1. Tautomerism of 2-mercaptobenzoylhydrazones of aliphatic aldehydes 2a-2h. Alk = H (2a), Me (2b), Et (2c), Bu (2d), i-Pr (2e), i-Bu ( 2f ), s-Bu (2g), t-Bu (2h).
![]()
Figure 2. Tautomerism of 2-mercaptobenzoylhydrazones of aromatic aldehydes 3a-3h. X = 4-NO2 (3a), 3-NO2 (3b), 4-Br (3c), 4-Cl (3d), H (3e), 4-Me ( 3f ), 4-MeO (3g), 4-Me2N (3h).
NMR spectrum. It is indicated by two signals of NH protons at 5.69 and 9.44 ppm and by a doublet at 4.29 ppm (H-2), and the 13C-NMR spectrum of this form is characterized by the signal of sp3-hybridized atom C-2 at 58.49 ppm. Gradually in the 1H-NMR spectrum of compound 2a in DMSOd6 signals appear corresponding to the stereoisomer of the linear acylhydrazone form A: one signal of azomethine protons at 7.43 ppm and also broadened signal of NH protons in the region 11.89 ppm. After some time the spectrum of compound 2a stopped to change indicating that the ring-chain equilibrium was attained where alongside the cyclic thiadiazepine form B existed one stereoisomeric modification of the linear form A. A similar situation exists for the solutions in DMSOd6 of other condensation products of aliphatic aldehydes with 2-mercaptobenzoic acid hydrazide 2b-2h (Table 1). Transition from compounds 2b-2d having an alkyl substituent with normal structure to compounds 2f-2h containing bulky isobutyl, sec-butyl, and tert-butyl groups leads to the appearance in the equili- brium of the second linear isomer and to the considerable decrease in the con- tent of cyclic tautomer B in the solution.
The observed doubling of the signals of the linear form A in the 1H-NMR spectra of compounds 2f-2h must be linked with the presence of conformational E',Z'-isomers, differing in the disposition of substituents relative to the amide C?N bond. The existence of an E,Z-configuration of isomer relative to the C=N bond was not considered by us since aldoacylhydrazones exist primarily or completely in the E-configuration relative to this bond [10] [11] [12] [13] . Assignment of the signals of the E,E'- and E,Z'-isomers of the linear form A was based on the known difference in position of the signals of the azomethine protons of the conformational E',Z'-isomers in the 1H-NMR spectra [14] . The signals of the Z'-isomer of this group are disposed at lower field than the analogous signals of the E'-isomer. An opposite value of both signals in the 1H-NMR spectra is observed for the protons of the NHCO groups of these conformers. Taking into consideration the above-indicated it may be confirmed that the main isomer has the E,Z'-structure, and the minor isomer the E,E'-spatial disposition.
![]()
Table 1. Tautomeric composition of the aliphatic aldehydes 2-mercaptobenzoyl-hydra- zones 2a-2h in DМSОd6.
*Tautomeric equilibrium constant KT = [B]/[E,E' − А] + [E,Z' − А].
The position of the equilibrium AB depends on the length and branching of the alkyl substituent: the introduction of bulky groups into the aliphatic aldehyde component leads to a displacement of the ring-chain equilibrium to the side of the 2-mercaptobenzoylhydrazone form A (Table 1). A linear correlation is then observed between the logarithms of the tautomeric equilibrium constants KT and the Taft ES constant [15] , [16] , [17] . The use of the Palm steric constants ES° [18] improves the correlation (Table 2 and Figure 1).
By an example of compound 2h we explored the effect of solvent on the tautomeric equilibrium (Table 3). In crystalline state compound exist as benzo- 1,3,4-thiadiazepine B. This proofs to be true because in its solid-phase 13C-NMR spectrum sp3-hybridized atom C-2 at 84.56 ppm is present (Experimental part). In low-polar CDCl3 about 70% of cyclic benzo-1,3,4-thiadiazepine form was detected. In going to basic dipolar solvents such as DMSOd6 and DMFd7 the domination of the linear form A was favored.
Thus the condensation products of aliphatic aldehydes with 2-mercaptoben- zoic acid hydrazide 2a-2h in the crystalline state possess the structure benzo- 1,3,4-thiadiazepine, and in solution of polar solvents they partially transform into the open-chain form A; consequently, it is only tentatively possible to use the name “mercaptobenzoylhydrazone” for such compounds.
In the series of aromatic aldehydes 2-mercaptobenzoylhydrazones 3a-3h the ring-chain tautomeric equilibrium should shift to the open-chain form A for the latter is stabilized by involving the aromatic ring in the system of π-p-π-conju- gation of the acylhydrazone fragment [10] [12] . This assumption was completely confirmed by the study of 1H- and 13C-NMR spectra of compounds 3a-3h (Figure 2).
![]()
Table 2. Correlation of the logarithms of the tautomeric equilibrium constants KT of compounds 2a-2h with substituent constants of Taft ES and Palm ESº according to the equation: lgKT = a + ρ ・ X; (n = 8).
![]()
Table 3. Tautomeric composition of trimethylacetic aldehyde 2-mercaptobenzoyl-hy- drazone 2h in different solvents.
In the 1H-NMR spectra of solutions in DMSOd6 of all the synthesized compounds 3a-3h there were signals corresponding both to the linear A and to the cyclic benzo-1,3,4-thiadiazepine B tautomeric forms. The signals of the linear 2-mercaptobenzoylhydrazone tautomer A were doubled in the spectra (Table 4). The observed doubling of the signals of the linear form A in the 1H-NMR spectra of compounds 3a-3h must be linked with the presence of conformational E',Z'-isomers, differing in the disposition of substituents relative to the amide C?N bond. An E, Z'-structure must therefore be attributed to the main isomer whereas E,E'-structural disposition to the minor isomer.
The existence of an E,Z-configuration of isomer relative to the C=N bond was not considered by us since aldoacylhydrazones exist primarily or completely in the E-configuration relative to this bond [10] , [12] . The assignment of signals belonging to E,E'- and E,Z'-isomers of linear form A was based on the known difference in the chemical shifts of the carbon atoms in the C=N and C=O bonds in the 13C-NMR spectra of conformational isomers of monocarbonyl compounds acylhydrazones; the E'-isomer signals of these groups were located in the region of 145 and 170 ppm, whereas those of Z'-isomer, at 150 and 160 ppm respectively [3] [12] [13] . In view of the above the major isomer of compounds 3a-3h had E,Z'-configuration, and the structural arrangement of the minor isomer was E,E'-configuration. The existence of the cyclic form B in solution may be judged in the 1H-NMR spectra by the doublet signals of the H-2 and NHCO protons at 5.76 and 9.62 ppm respectively and also by the doublet-doublet signals of the NH group proton at 6.25 ppm, which is caused by a spin-spin interaction with the protons in positions 2 and 4 of the benzo-1,3,4-thiadiazepine cycle (Experimental part).
The introduction of an electron-withdrawing substituent into the aromatic ring of the aldehyde component leads to a displacement of the ring-chain equilibrium AB to the side of the cyclic benzo-1,3,4-thiadiazepine form B, and a linear correlation is then observed between the logarithms of the tautomeric equilibrium constants KT and the Hammett σ-constant [16] [19] . The use of the σ+-constant of Brown [20] improves the correlation (Table 5 and Figure 3). The
![]()
Table 4. Tautomeric composition of the aromatic aldehydes 2-mercaptobenzoyl-hydra- zones 3a-3h in DМSОd6.
*Tautomeric equilibrium constant KT = [B]/[E,E' −А] + [E,Z' − А].
![]()
Figure 3. Plots of the tautomeric equilibrium KT logarithms correlation of compounds 2a-2h and 3a-3h respectively with substituent constants: of Palm 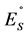 alkyl substituent constants (left), of Brown σ+ substituent constants (right).
alkyl substituent constants (left), of Brown σ+ substituent constants (right).
![]()
Table 5. Correlation of the logarithms of the tautomeric equilibrium constants KT of compounds 3a-3h with substituent constants of Hammett σ and Brown σ+ according to the equation: lgKT = a + ρ ∙ X; (n = 8).
same regularity is explained by the fact that the electron-withdrawing substituents strengthen the electrophilicity of the carbon atom of the C=N bond, addition of the SH function to which leads to the formation of benzo-1,3,4-thiadia- zepine tautomer B. The conformational equilibria within the linear tautomer are sensitive to a lesser extent to the nature of the substituent in the aromatic ring. Probably in both linear forms E,E'-A and E,Z'-A identical systems of conjugation occur, reacting in the same way to the change of electronic parameters of the substituent (Table 4).
In a much greater extent the position of the tautomeric equilibrium is depen- dent on the nature of solvent used. This was demonstrated by the example of 4- chlorobenzoic aldehyde 2-mercaptobenzoylhydrazone 3d (Table 6). The open- chain structure E,Z'-A of compound 3d in crystalline state was confirmed by its solid-phase 13C-NMR spectrum: 150.74 (C=N), 166.55 (C=O) ppm (Experimental part). The same form was detected under the study of the spectrum of compound 3d taking of in CDCl3. In going to basic dipolar solvents such as DMSOd6 and DMFd7 the domination of the benzo-1,3,4-thiadiazepine form B was favored. Perhaps, its stabilization is due to the possibility of hydrogen bonds formation between the NH groups of the benzo-1,3,4-thiadiazepine ring and the polar solvent molecules.
3. Conclusions
Therefore, in contrast to the products of the condensation of aldehydes with the hydrazides of 2-hydroxy- and 2-aminobenzoic acids reported in the literature [8] [9] , 2-mercaptobenzoylhydrazones of aliphatic and aromatic aldehydes display a tendency to cyclize to give the seven-membered 1,3,4-benzothiadiazepine
![]()
Table 6. Tautomeric composition of 4-chlorobenzoic aldehyde 2-mercaptobenzoyl-hy- drazone 3d in different solvents.
ring. This naturally reflects the greater nucleophilicity of the sulfur atom of the SH function, which participates in intramolecular cyclization, in comparison with the nucleophilicity of the oxygen and nitrogen atoms in the OH and NH functions in the hydrazones obtained using 2-hydroxy- and 2-aminobenzoic acid hydrazides. In this regard, 2-mercaptobenzoylhydrazones are similar to the products of the condensation of carbonyl compounds with thiobenzoic and thioglycolic acid hydrazides, for which intramolecular attack by the sulfur atom at the C=N bond of the hydrazone fragment leads to formation of 1,3,4-thiadi- azoline [21] and 1,3,4-thiadiazine rings [7] [12] , respectively. The determination of the conformational state of the seven-membered 1,3,4-benzothiadiazepine ring requires further study.
4. Experimental Part
4.1. General Methods
1Н-and 13С-NMR spectra were registered on a spectrometer Bruker AV-400 at operating frequencies 400 and 100 MHz respectively (internal reference hexamethyldisiloxane). The solid-phase 13C-NMR spectra were obtained on a Bruker AM-500 spectrometer (125 MHz) using a standard procedure utilizing cross polarization and magic angle spinning (CPMAS) technique (frequency 4.5 kHz; internal reference hexamethylbenzene). The tautomeric composition of obtained compounds was estimated by the integration of the appropriate signals in the 1Н NMR spectra, and error of measurement was ±1%. Elemental analysis of previously unknown compounds was carried out on a CHN Analyzer Hewlett Packard 185B. The purity of prepared compounds was checked by TLC on Silufol UV- 254 plates, eluent benzene-acetone, 4:1. 2-Mercaptobenzoic acid hydrazide 1 was obtained by method [22] .
4.2. Synthesis of 2-Mercaptobenzoylhydrazones 2 and 3 (General Procedure)
To a solution of 2-mercaptobenzohydrazide 1 (1.68 g, 10 mmol) in methanol (25 ml) an appropriate aldehyde (11 mmol) was added (for compound 2a 1.0 ml of 40% formaline was used), and the mixture was kept at room temperature for a period of 10 - 15 min. The solvent was removed at a reduced pressure, and the residue was washed with ether (3 × 25 ml), filtered off, dried and recrystallizated.
Physico-chemical and spectral characteristics of compounds 2b-2f and 3a-3c, 3e-3h were described previously [23] [24] .
4.3. Formic Aldehyde 2-Mercaptobenzoylhydrazone (2a)
Yield 54%, m.p. 201˚ - 203° (acetonitrile) (m.p. [22] 203˚ - 206˚). 1Н-NMR spectrum (DMSOd6): δ = form E,Z'-А (0.5%): 11.89 (br.s, NHCO), 7.43 (br.s, HC=N); form B (99.5%): 4.29 (d, J = 7.8 Hz, Н-2), 5.69 (dt, J1 = 7.8 Hz, J2 = 5.0 Hz, NH), 7.47 - 7.60 (Ar in E,Z'-A and B), 9.44 (d, J = 5.0 Hz, NHCO) ppm. 13C-NMR spectrum (DMSOd6): δ = form B: 58.49 (С-2), 128.42 - 140.96 (Ar), 173.60 (С-5) ppm. Found, %: C 53.26; H, 4.53; N, 15.49. C8H8N2OS. Calculated, %: C 53.31, H 4.47, N 15.54.
4.4. 2-Methylbutyric Aldehyde 2-Mercaptobenzoylhydrazone (2g)
Yield 63%, m.p. 88˚C - 91˚C (benzene - hexane, 1:4). 1H-NMR spectrum (DMSOd6): δ = form E,E'-А (6%): 0.86 (t, J = 7.4 Hz, CH3CH2), 0.97 (d, J = 6.5 Hz, CH3CH), 1.06 (m, CH3CH2), 1.40 (m, CH3CH), 7.44 (d, J = 4.2 Hz, HC=N), 11.98 (br.s, NHCO), form E,Z'-А (38%): 0.86 (t, J = 7.4 Hz, CH3CH2), 0.97 (d, J = 6.5 Hz, CH3CH), 1.21 (m, CH3CH2), 1.51 (m, CH3CH), 7.60 (d, J = 4.2 Hz, HC=N), 11.57 (br.s, NHCO), form B (56%): 0.84 (t, J = 7.2 Hz, CH3CH2), 0.90 (d, J = 6.4 Hz, CH3CH), 1.65 (m, CH3CH), 1.75 (m, CH3CH2), 4.43 (dd, J1 = 7.5 Hz, J2 = 5.5 Hz, H-2), 5.74 (dd, J1 = 5.5 Hz, J2 = 5.0 Hz, NH), 7.42 - 7.62 (Ar in A and B), 9.44 (d, J = 5.0 Hz, NHCO) ppm. 13C-NMR spectrum (DMSOd6): δ = form E,E'-A: 11.43 (CH3), 17.14 (CH3), 26.97 (CH2), 166.89 (C=O), form E,Z'-A: 11.54 (CH3), 16.19 (CH3), 25.59 (CH2), 37.85 (CH), 156.91 (C=N), 163.07 (C=O), form B: 10.95 (CH3), 15.32 (CH3), 26.17 (CH2), 38.59 (CH), 78.72 (C-2), 128.17 - 140.39 (Ar in A and B), 173.16 (C-5) ppm. Found, %: C 60.94; H 6.88; N 11.90. C12H16N2OS. Calculated, %: C 60.99; H 6.82; N 11.85.
4.5. Trimethylacetyc Aldehyde 2-Mercaptobenzoylhydrazone (2h)
Yield 57%, m.p. 132˚C - 134˚C (benzene - hexane, 1:4). 13C-NMR spectrum (solid phase): δ = form B (100%): 27.12 ((CH3)3C), 35.07 ((CH3)3C), 84.56 (C-2), 126.84 - 142.21 (Ar), 174.79 (C-5) ppm. 1H-NMR spectrum (DMSOd6): δ = form E,E'-A (12%): 0.95 (s, (CH3)3C), 7.60 (s, HC=N), 11.81 (br.s, NHCO), E,Z'-A (61%): 1.11 (s, (CH3)3C), 7.65 (s, HC=N), 11.54 (br.s, NHCO), form B (27%): 0.97 (s, (CH3)3C), 4.40 (d, J = 9.5 Hz, H-2), 5.63 (dd, J1 = 9.5 Hz, J2 = 5.0 Hz, NH), 7.34 - 7.66 (Ar in A and B), 9.41 (d, J = 5.0 Hz, NHCO) ppm. 13C-NMR spectrum (DMSOd6): δ = form E,E'-A: 27.06 ((CH3)3C), 36.80 ((CH3)3C), 147.86 (C=N), 163.04 (C=O), form E,Z'-A: 27.06 ((CH3)3C), 36.80 ((CH3)3C), 150.43 (C=N), 159.88 (C=O), form B: 27.24 ((CH3)3C), 34.72 ((CH3)3C), 84.22 (C-2), 126.24 - 140.33 (Ar in A and B), 173.00 (C-5) ppm. Found, %: C 61.03; H 6.76; N 11.91. C12H16N2OS. Calculated, %: C 60.99; H 6.82; N 11.85.
4.6. 4-Chlorobenzoic Aldehyde 2-Mercaptobenzoylhydrazone (3d)
Yield 76%, m.p. 181˚C - 183˚C (ethanol). 13C-NMR spectrum (solid phase): δ = form E,Z'-A (100%): 125.00 - 132.21 (Ar), 150.74 (C=N), 166.55 (C=O) ppm. 1H- NMR spectrum (DMSOd6): form E,E'-A (9%): 8.12 (s, HC=N), 12.13 (br.s, NHCO), form E,Z'-A (56%): 8.40 (s, HC=N), 11.96 (br.s, NHCO), form B (35%): 5.76 (d, J = 6.9 Hz, H-2), 6.25 (dd, J1 = 6.9 Hz, J2 = 3.8 Hz, NH), 7.38 - 7.76 (Ar in A and B), 9.62 (d, J = 3.8 Hz, NHCO) ppm. 13C-NMR spectrum (DMFd7): δ = form E,E'-A: 141.21 (C=N), 173.28 (C=O), form E,Z'-A: 147.00 (C=N), 164.82 (C=O), form B: 73.95 (C-5), 173.90 (C-5), 124.79 - 139.10 (Ar in A and B) ppm. Found, %: C 57.78; H 3.77; N 9.79. C14H11ClN2OS. Calculated, %: C 57.83; H 3.81; N 9.63.