Impact of Cardamom Cultivation on the Composition and Dynamics of Soil Seed Banks in a Conservation Forest in Sri Lanka: Implications for Conservation ()
1. Introduction
Agro-ecosystems are important elements of many tropical landscapes, as they contribute to local livelihoods and provide environmental services. As in other areas in the tropics where perennial crops have been integrated with forest trees ( Bhagwat et al., 2008 ; Parthasarathy, 1999 ; Pascarella et al., 2000 ; Rivera et al., 2000 ; Rivera & Aide, 1998 ), in parts of Asia many forests have been cultivated with the high-value spice crop cardamom (Elettaria cardamomum (L.) Maton) ( Buckingham, 2004 ; Kumar et al., 1995 ; Reyes et al., 2006 ). Despite its socio- economic benefits, the system of cardamom cultivation in tropical Asian forests has been criticised for being incompatible with conservation of forest resources ( Ashton et al., 2001 ; Dhakal et al., 2012 ; Gunawardane, 2003 ). Cardamom cultivation and management involves the selective removal of canopy trees and regular maintenance by removal of competing plants in the forest understorey ( Anon, 2002 ; Bandaratillake, 2005 ; Buckhingham, 2004 ; Kumar et al., 1995 ). This system of cultivation may hinder the capacity of forests to regenerate and undermine their conservation value ( Ashton et al., 2001 ). Therefore, research on restoration techniques for high conservation value forests affected by repeated cardamom cultivation should be a high priority to conserve biodiversity and ecosystem services they support.
Restoration of forests following disturbance hinges on understanding the pathways by which plant species colonise the sites and the constraints on recruitment of late successional tree species. The soil seed bank represents an important source of propagules for regeneration of forests following agricultural use ( Decocq et al., 2004 ; Pickett & McDonnell, 1989 ; Quintana-Ascencio et al., 1996 ; Saulei & Swaine, 1988 ; Swaine & Hall, 1983 ; Thompson, 1992 ; Uhl et al., 1981 ). A comparison of soil seed banks between undisturbed and adjacent degraded forests may contribute to understanding the impacts of disturbance on the density and community structure of buried seeds, and the capacity of soil seed banks to contribute to forest recovery and/or inhibit tree regeneration. Although the soil seed bank is not likely to represent an important source of propagules for regeneration of late-successional trees ( Tekle & Bekele, 2000 ), it may contain a higher density of plant groups, such as pioneer trees, grasses, forbs and non-native species, which may impact future forest composition through competition with newly-emerged tree seedlings ( Denslow et al., 2006 ). Thus this assessment contributes baseline knowledge for designing approaches to the restoration and management of disturbed forests ( Butler & Chazdon, 1998 ; Lemenih & Teketay, 2006 ; Martins & Engel, 2007 ; Richter & Stromberg, 2005 ; Wang et al., 2009 ). Several studies have documented a higher density of seeds, a higher proportion of early successional species and a greater species diversity in the seed banks of tropical forests disturbed by shifting cultivation or logging than in adjacent undisturbed forests ( Alvarez-Aquino et al., 2005 ; Arevalo & Fernandez-Palacios, 2000 ; Dalling & Denslow, 1998 ; Leckie et al., 1999 ; Osumi & Sakurai, 1997 ; Quintana-Ascencio et al., 1996 ; Saulei & Swaine, 1988 ; Sousa et al. 2017 ). To our knowledge no studies have investigated the effects of cultivating a perennial crop in the understorey of a tropical forest on the density and composition of soil seed banks and their implications for forest regeneration and the future structure of the tree community.
The cultivation of cardamom in the understorey of lower montane rain forests (altitude between about 1000 m and 1400 m) in Sri Lanka leads to a reduction in the density of trees (≥5 cm DBH) and an increase in the relative abundance of light-demanding trees that form the forest canopy ( Dhakal et al., 2012 ). These changes generate three predictions in terms of their impacts on the density and composition of the soil seed bank. First, the reduction in stem density may increase canopy openness and thereby light transmission to the forest floor ( Ku- mar et al., 1995 ; Parthasarathy, 1999 ). An increase in light availability may sti- mulate the development of an herbaceous ground flora including non-native species comprising both grasses and forbs ( Garwood, 1989 ; Leckie et al., 1999 ). Over time, these herbaceous species may come to dominate the soil seed bank of disturbed forests and contribute significantly to an increase in the overall density of buried seeds ( Lemenih & Teketay, 2006 ). An increase in the abundance of grasses, forbs and non-native species in soil seed banks would be important if these species impede regeneration of late-suecessional trees through inhibition of tree seedlings, especially once the canopy has been opened ( Denslow et al., 2006 ; Guariguata & Ostertag, 2001 ).
Secondly, the increase in relative abundance of light-demanding species in the tree canopy may be reflected in a parallel increase in the density of seeds of these species in the soil seed bank. In lower montane rain forest in the Knuckles Forest Reserve of Central Sri Lanka, the pioneer tree Macaranga indica Wight. has a substantially greater relative abundance in abandoned cardamom plantations than in adjacent undisturbed natural forest ( Dhakal et al., 2012 ). As seeds of other Macaranga species, such as M. Peltata Roxb., and M. Heynei I. M. Johnst. are an important component of the soil seed banks of Asian tropical and subtropical forests ( Chandrashekara & Ramakrishanan, 1993 ; Lin et al., 2006 ; Metcalfe & Turner, 1998 ), we predict that seeds of M. indica will be present in the soils that we sampled, but at much higher densities in the soils of abandoned cardamom plantations than adjacent natural forests.
Thirdly, it has been documented that when a landscape undergoes extensive change as a result of human activities, plant communities become more susceptible to invasion by non-native plant species ( Hansen & Clevenger, 2005 ). In some cases, these non-native species may increase in abundance in the soil seed banks of forests disturbed by human use ( Conn et al., 1984 ; Lin et al., 2006 ; Moffatt et al., 2004 ; Peterson & Carson, 1996 ). Hence, we test the prediction that forests disturbed by cardamom cultivation manifest a greater abundance and species richness of non-native species in the soil seed bank than in adjacent undisturbed natural forests.
The aim of this study was to determine how the composition and structure of the soil seed bank responds to the introduction of a cardamom crop in a high conservation value lower montane rain forest in Sri Lanka. This research underpins the development of strategies for restoration and conservation of forests containing abandoned cardamom plantations in the buffer zone of a globally significant World Heritage Site. Data on the ecological impacts of cultivating perennial cash crops beneath a closed tree canopy are scarce, despite the widespread occurrence of analogous agroforest-ecosystems involving crops such as coffee, cocoa and ginger ( Bhagwat et al., 2008 ; Schroth & da Mota, 2013 ). Specifically, by comparing the density and composition of soil seed banks in abandoned cardamom plantations to those in adjacent undisturbed forests, we tested the following predictions: 1) the density of seeds in the soil seed bank has increased in response to disturbance associated with cultivation in the abandoned cardamom plantation; 2) this difference is associated with a rise in the abundance of herbaceous plants in the seed bank of the disturbed forest; 3) seeds of light-demanding pioneer trees such as Macaranga species are present at a higher density in the soil of the abandoned cardamom plantation, reflecting their greater abundance in the above-ground tree community; and 4) seeds of non- native species are present at a higher density in the soil of the abandoned cardamom plantation than in the soil of undisturbed forest.
2. Materials and Methods
2.1. Study Site
This study was undertaken in the Knuckles Forest Reserve (KFR, 7˚21'N to 7˚24'N, 80˚45'E to 80˚48'E), which extends over 21,000 ha in the central highlands of Sri Lanka ( Balasubramaniam, 1988 ; Bambaradeniya & Ekanayake, 2003 ). The KFR contains a range of natural vegetation types reflecting steep gradients of altitude and climate, as well as patches of human-induced grassland, areas of shifting cultivation, and plantations of Pinus caribaea Morelet ( Bambaradeniya & Ekanayake, 2003 ). The study site was located in lower montane rain forest at 1032 - 1274 m close to the settlement of Riverston (7˚31'N, 80˚44'E) in the north-eastern part of KFR (Figure 1). The mean monthly rainfall at the study site ranges from 93 mm (May) to 673 mm (January) ( MSP, 2009 ). The mean annual temperature ranges from 18˚C to 26˚C ( Gunaratne, 2007 ). The dominant tree species forming the canopy in the cardamom plantations are Macaranga indica, Neolitsea cassia (L.) Kosterm., Elaeocarpus glandulifer (Hook. Ex Wright) Mast., Turpinia malabarica Gamble, and Symplocos cochinchinensis (Lor.) Moore var laurina, and in the natural forests the most common canopy tree species are Calophyllum trapezifolium Thwaites., Syzygium operculatum (Roxb.)
![]()
Figure 1. Map of Knuckles Forest Reserve, Sri Lanka, showing different areas and land use types.
Nied., Schefflera sp., and Psychotria thwaitesii Hook. f. ( Dhakal et al., 2012 ).
Cardamom is a perennial spice crop originating in Asia and parts of Africa ( Buckhingham, 2004 ). It is a perennial herb with pseudo stems (technically leaves) extending to 2 - 3 m in height from a fleshy branched rhizome. Cardamom displays an optimum productivity when grown in about 50% shade and establishes well under the canopy of tropical montane forests. Cardamom gains reproductive maturity three years after planting, and has an economic life of 10 - 15 years ( Nybe et al., 2007 ). Pods or seed capsules grow low to the ground and contain the black, green or white seeds. The seed capsules are highly aromatic, which explains their use as a spice and in traditional medicine as a tonic or infusion.
Official records suggest that cardamom has been planted into about 3000 ha of natural montane forests within the KFR ( Gurusinghe, 1988 ; IUCN, 1994 ). However, this figure is likely to be an underestimate of the total area under cardamom cultivation because we observed very few patches of natural montane forest that lacked cardamom in the understorey during three years of research. Cardamom cultivation in the forest started more than a century ago ( Guru- singhe, 1988 ) and the plantations were managed continuously until the Knuckles Conservation Area (KCA) was established in 1985. At that time, formal management of the cardamom plantations ceased because cultivation within the KCA was prohibited ( Anon, 2000 ; IUCN, 1994 ). By that time cardamom had been planted into most of the suitable forest, and remaining patches of natural forest without cardamom were small and widely scattered, including some areas where there was conflict over land ownership. The average (± SE) density of cardamom plants in the abandoned cardamom plantations at the time of this study was 7525 (±973) ha−1.
2.2. Plot Selection and Sampling of the Soil Seed Bank
Plots (10 m × 10 m) established for tree regeneration studies ( Dhakal, 2011 ) were used for locating samples in this study. Forty-eight plots were sampled in cardamom plantation (CP), derived from eight blocks of each six contiguous plots, and eight non-contiguous plots were sampled in natural forest without cardamom (NF). The maximum distances between blocks and between CP and NF plots were 1.4 km and 1.8 km, respectively.
Two soil samples (10 cm × 10 cm × 5 cm deep) were collected from locations selected at random within 50 cm of the edge of two opposite sides of each plot and mixed thoroughly to make a composite sample of 20 cm × 10 cm × 5 cm. This procedure yielded 48 samples from CP and eight samples from NF. Sampling was repeated on two occasions: 15 August 2008 (end of dry season) and 26 February 2009 (end of wet season). On the day of collection, soil samples were spread out in polythene trays to a depth of 5 mm on the surface of sterilised sand (4 cm deep) in a shade house at 550 m a.s.l. Two trays 40 cm × 25 cm × 5 cm per sample were used to accommodate each sample and the trays were placed randomly in the shade house. The sand was steam-sterilised uniformly to kill contaminating seeds. For each set of samples, eight additional heat-sterilized soil samples were also spread over sand in trays and monitored to determine the extent of contamination by seeds blown or carried into the shade house. Four seedlings representing three morphotypes (individuals with similar morphology, but in some cases and unknown species identity)―one Cyperus, one Biophytum and one unknown, and three seedlings of three morphotypes―one Hedyotis and two unknown emerged from these control samples during the study of dry and wet season seed banks, respectively. As these numbers represented a very small percentage of total seedling emergence (<0.08%), contamination was ignored during data analysis.
All trays were watered regularly and germination was recorded weekly for 19 weeks, which represented approximately 2 weeks since germination had ceased in all cases. Emergents were counted and marked using toothpicks, and categorised into different morphotypes. The trays were randomly relocated twice a month to reduce the difference in environmental conditions for replicate trays during the study period. After 16 or 17 weeks, the soil was turned over to promote the germination of deeply buried seeds. Herbarium specimens of each morphotype were prepared and identified by comparison with specimens in the National Herbarium, Peradeniya (PDA).
2.3. Measurement of Canopy Openness and Standing Vegetation
Canopy openness in the forest was estimated from hemispherical photographs (Nikon Coolpix 995 camera body with a FC-E8 0.21x Nikon fisheye lens, Nikon Corporation, Japan) taken at the centre of each 10 m × 10 m plot between 6 a.m. and 8 a.m. to avoid direct sunlight in the field of view. The camera with hemispherical lens was placed in a self-levelling camera mount at a height of 1.4 m above ground level, and oriented to true north while taking photographs. The hemispherical photographs were converted to black and white images by using Adobe Photoshop (2008 Adobe Systems Incorporated, USA), and percentage canopy openness estimated by using the software WINPHOT 5.0 ( ter Steege,, 1996 ).
To compare the species composition of the seed bank with that of the standing tree community in each plot, all trees ≥ 5 cm DBH in each 10 m × 10 m plot were recorded and identified following the same procedure used to identify the species in the soil seed bank ( Dhakal, 2011 ). Although the soil samples were collected up to 50 cm outside opposite edges of these plots, seed dispersal limitation would not be sufficient at this scale to represent a severe constraint to comparison of below- and above-ground plant communities.
2.4. Micro-Climatic Measurements
Sensors attached to a Skye Data Hog 2 (Model SDL 5490, Skye Instruments Ltd, UK) were used to measure photosynthetically active radiation (PAR; SKP 215), air temperature (SKTS 200U/I), relative humidity (SKH 2040/I), and soil temperature (SKTS 200/I) in the shade house and in plots with abandoned cardamom and in natural forests during April-June 2009 to provide contextual information.
The sensors inside the shade house were located adjacent to the germination trays whereas in the cardamom plantations and natural forests they were placed in the plots where trees were censused. The soil temperature sensor inside the shade house was buried to a depth of ca. 4 cm, in one of the seed trays. Photosynthetically active radiation and air temperature were recorded 10 cm above ground, and soil temperature in the forests was recorded at 4 cm below ground. The air temperature sensor was covered by a white plastic cup to avoid direct heating by solar radiation. Data from all sensors were collected at 10-second intervals and averaged over 30 minutes for five consecutive days in each location.
The mean (±SE) values inside the shade house, in an abandoned cardamom plantation and in a natural forest, respectively, were as follows: PAR, 5.13 ± 0.33, 11.2 ± 1.99, and 8.30 ± 1.81 mol m−2 day−1; soil temperature, 24.4˚C ± 0.05˚C, 20.9˚C ± 0.03˚C, and 20.5˚C ± 0.06˚C; air temperature, 23.7˚C ± 0.09˚C, 20.6˚C ± 0.10, and 20.8˚C ± 0.13˚C; and relative humidity, 80.5% ± 0.45%, 91.5% ± 0.31%, and 92.7% ± 0.10%. Statistical analysis of these data was not possible because the measurements were not made simultaneously and spatial variance within sites was not determined, but they suggest that the seed trays may have been exposed to lower PAR and relative humidity, and higher soil and air temperature than forest floor environments that were sampled for soil seed banks. However, the conditions in the shade house are unlikely to have been limiting for seed germination based on published information for tropical plants (Baskin & Baskin 1998), and we do not consider that these differences therefore bias our conclusions.
2.5. Data Analysis
The mean (±SE) density of emergents per square metre was calculated for cardamom plantations (CP) and natural forests (NF) for both dry and wet seasons and for the pooled data from both seasons. To account for the nesting of plots within blocks of the CP, generalised linear mixed models (GLMM) implemented using the glmmPQL function in the MASS package of R v. 1.11.1 ( R Core Development Team, 2010 ) were fitted to counts of seeds emerging from the soil seed bank. This function fits a generalised linear mixed model with multivariate random effects, using penalised quasi-likelihood ( Zuur et al., 2009 ). Models fitting counts of seeds included a log link function and tested the contrasts in seed density in the soil seed bank between abandoned cardamom plantations and natural forests and between wet and dry seasons with count of seeds emerging from the soil seed bank as a fixed effect, and the plots (including nested within block) as random effects. In all cases the full model including all main effects and their interactions was fitted first and then simplified by sequential removal of the non-significant terms. Graphical methods were used to assess how well the models fit the data. The mean density per sample of non-native species in the soil seed banks of CP and NF during dry and wet seasons and in the data pooled across both seasons were analysed by using analogous models. The relative abundance of non-native species and the counts of seedling emergents in different life-form categories were compared between CP and NF for each season, and between seasons for data pooled across both forest types, using Chi-square tests.
Species richness of seeds in the seed banks of each forest type for dry and wet seasons, and in the pooled data, were estimated using EstimateS 7.5.2 ( Colwell, 2006 ). Species accumulation curves were obtained by plotting cumulative species number against the cumulative number of individuals (individual-species curves) and comparisons of species richness were made for a standard sample of 400 emergents, which is the maximum number obtained for the smaller of the two samples. Fisher’s alpha diversity index ( Williams, 1947 ) was used for estimation of species diversity because it is relatively insensitive to sample size, and to the abundance of the most common species ( Dalling & Denslow, 1998 ). Values of Fisher’s alpha for buried seeds were compared between CP and NF using analogous GLMM models. The total number of species per sample was compared between CP and NF for dry and wet seasons and for the pooled data across both seasons using the GLMM models with values of alpha diversity as fixed effects and the plots as random effects. The species evenness (equitability) was calculated using the values of Simpson’s index estimated using Estimate S and the species richness was compared between CP and NF using GLMM models with evenness as fixed factor and plots as fixed effects.
We described the patterns of community composition in soil seed banks using non-metric multidimensional scaling (NMS) in PC-ORD ( McCune & Grace, 2002 ). The NMS ordinations were conducted using the Sorensen distance measure with 50 runs of real data and 50 runs of randomised data to generate a Monte Carlo test of significance ( McCune & Grace, 2002 ). The final stress of the ordination and coefficients of determination (R2) are reported for each ordination axis as a proportion of the variation explained. A secondary matrix comprising site environmental variables (elevation and canopy openness) was overlaid on the NMS ordinations to aid interpretation of the distribution of samples and species. The strength and direction of the association of each environmental variable with variation in tree species composition are indicated by a vector in ordination space such that the length of the vector represents the rate of change in the weighted average as inferred from the biplot, and indicates how much the species distributions and sites differ along that environmental variable. The most important variables are those with the longest vector. In order to reduce the “noise” ( McCune & Grace, 2002 ) from infrequent species, species that were observed only once or twice were excluded from the NMS analysis. The data used in the secondary matrix were relativised (by general relativisation) since canopy openness was expressed on a percentage scale ( McCune & Grace, 2002 ). In order to analyse the patterns in soil seed bank across seasons and forest types, the data were pooled across seasons in CP and NF prior to the NMS analysis.
The differences in species composition of seed banks between CP and NF were also examined separately for dry and wet seasons and for data pooled across both seasons, using the multi-response permutation procedure (MRPP) ( McCune & Grace, 2002 ), which derives a test statistic, T, and a P value from a non-parametric multivariate test of differences among a priori groups. A more negative value for T indicates a stronger separation between groups ( Elliott & Swank, 2008 ). The Sørenson (Bray-Curtis) index was used as a distance measure for the MRPP analysis.
A comparison of the species composition of the seed bank with that of the tree (≥5 cm DBH) community (standing vegetation) was conducted using Sørenson’s similarity index for presence and absence data (Cs):
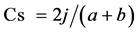
where j is the number of species common to both the seed bank and tree communities, a is the number of species in the seed bank, and b is the number of species in the tree community ( Dalling & Denslow, 1998 ). The proportion of overstorey species that were represented in soil seed banks in each forest type was calculated and analysed by using Chi-square tests.
3. Results
3.1. Density of Seeds in the Soil
The density of seeds was significantly higher in the soil of the abandoned cardamom plantation than the natural forest (Table 1; Figure 2). A similar pattern was observed in the dry season and the wet season in CP and NF, respectively
![]()
Table 1. Mean values (±SE) of species richness per sample, diversity and evenness and their statistical significance in the soil seed banks of cardamom plantation (CP) and natural forest (NF) during dry and wet seasons, and in data pooled across both seasons. The statistical significance is evaluated using P value, considering value < 0.05 as significant. In order to avoid pseudo-replication, the GLMM models were used while analysing the data. The estimated numbers of species per sample of 400 seeds (with 95% CI in parentheses) and the values of Sørensen’s similarity index are also given.
![]()
Figure 2. The density of germinants of trees, shrubs, forbs, grasses and others (unknowns, climbers and ferns) that emerged from soil taken from cardamom plantation (CP) and natural forest (NF) during dry and wet seasons. Different letters for life forms signify differences in densities between cardamom plantation and natural forest in two seasons at P ≤ 0.05. The data were analysed using GLMM.
(Table 1). The overall seed density was higher (t = −4.93, df = 110, P < 0.001) in the dry season (5420 ± 317 m−2) than in the wet season (3175 ± 299 m−2) (Figure 2). The interaction between forest types and season on density of seeds in the soil was not significant (t = 0.49, df = 108, P > 0.05).
3.2. Life Form Distribution
Grasses represented a greater proportion of buried seeds in the CP than in the NF, in both the dry (22% vs. 18% respectively) and wet (11% vs. 5%) seasons (Figure 3). Forbs contributed the highest number and percentage of emergents in both forest types in both seasons (Figure 3). The proportion of tree seeds in the soil seed bank was higher in CP (25%) than in NF (13%) in the wet season, but it was lower in CP (14%) than in NF (16%) in the dry season (Figure 3). The proportional representation of different life-forms in the soil seed bank differed between dry and wet seasons in both CP (t = 6.18, df = 94, P < 0.001) and NF (t = 4.10, df = 14, P < 0.001), and between CP and NF for the combined data-set across both seasons (Figure 3; t = 5.35, df = 110, P < 0.001).
3.3. Composition and Richness of the Soil Seed Bank
Emergents from the 48 soil samples taken from the CP represented 72 species in 30 families, whereas in the eight samples collected in the NF there were only 49 species in 24 families (Table A1; supplementary data). The season-wise counts were different: in the CP the respective counts in the dry/wet seasons were, 66/44 species in 30/21 families; in the NF they were 36/34 species in 20/20 families, which highlights a greater seasonal variation in the number of taxa in soils taken from the CP (Table A1).
Species richness per sample of the soil seed bank was higher in CP than in NF
![]()
Figure 3. The percentages of trees, shrubs, forbs, grasses and others (unknowns, climber and fern species) in the soil seed banks of cardamom plantations (CP) and natural forests (NF) during dry and wet seasons.
![]()
Figure 4. Species accumulation curves against cumulative number of individual sampled for emergents from the soil seed banks of cardamom plantations and natural forests for dry (a) and wet (b) seasons, and pooled data from both seasons (c). Mean species richness was estimated using the computer program EstimateS 8.0 ( Colwell, 2006 ).
in the dry season, but not in the wet season. The pooled data across both seasons did not show any difference in species richness of the seed banks between the two forest types (Table 1). The rarefaction curves also suggest that species richness for a sample of 400 emergents was not different between CP and NF, either in the dry or the wet season (Figure 4(a), Figure 4(b)), or in the data pooled across both seasons (Figure 4(c)). Mean values of Fisher’s alpha diversity in the CP in the dry season were higher and in the wet season lower than in the NF (Table 1). For the pooled data, the mean values of Fisher’s alpha were lower in CP than in NF. The mean values of species evenness (equitability) of the soil seed bank were higher in the NF than in the CP in both dry and wet seasons and in the pooled data (Table 1).
In the soil seed bank of the CP, the most abundant species was Hedyotis nitida Wright and Arn. (Rubiaceae), which represented about 20% of all germinants (Table A1). In contrast, the most abundant species in the soil seed bank of NF was Oldenlandia corymbosa Linn. (Rubiaceae) which represented about 10% of all germinants (Table A1). The light-demanding species Macaranga indica, M. peltata and Symplocus cochinchinensis emerged only in soils taken from the CP. Macaranga indica represented 0.4% and 1.9% of all germinants in the dry and wet seasons, respectively.
The non-metric multidimensional scaling (NMS) ordination recommended three dimensions for the final solution. In the combined NMS analysis (data sets from both CP and NF sampled in two seasons), the final stress for the three dimensional solution was 18.9, which is acceptable for a reliable solution ( McCune & Grace, 2002 ). The proportions of variance explained were 31% for Axis 1, 26% for Axis 2, and 19% for Axis 3 (cumulative R2 of 76% for the first three ordination axes, Table 2). The two-dimensional plot representing the first two axes suggested that the samples were clustered in the ordination space, according to whether the samples were taken from CP or NF and whether sampling was conducted in the wet or dry season (Figure 5; Table 2). There was relatively little overlap among these four clusters, which suggests that the species composition of samples from each forest type and season was distinct. Samples from CP tended to have lower values along Axis 1 than those from NF in both seasons, which reflects a negative association between Axis 1 and canopy openness of the plot where the samples originated. Samples taken in the wet season tended to have higher values along Axis 2 than dry season samples for both forest types (Figure 5).
The light-demanding species M. indica, Acronychia pedunculata (L.) Miq., and S. cochinchinensis had relatively low values along Axis 1, which implies they were more strongly associated with samples from CP and plots with a higher canopy openness. Macaranga peltata was differentiated from M. indica by having a higher score along Axis 1 (Figure 6, Table A2).
![]()
Table 2. Correlations of canopy openness and elevation with the first three axes of a non-metric multidimensional scaling ordination of seedlings emergence data from cardamom plantations and natural forests sampled in wet and dry seasons in the Knuckles Forest Reserve. Pearson’s parametric (r) and Kendall’s non-parametric (tau) correlations with ordination axes (n = 112) are presented, with values of r ≥ 0.30 highlighted in bold. Monte Carlo Test for stress in real data was P < 0.05 for all axes shown.
![]()
Figure 5. Non-metric multidimensional scaling ordination diagram of plot scores using combined emergence data for seed banks sampled from cardamom plantations (CPD and CPW) and natural forests (NFD and NFW) in dry (CPD and NFD) and wet seasons (CPW and NFW). The environmental variable associated with the distribution of plots was canopy openness (Cop).
The multi-response permutation procedure (MRPP) analyses suggested that there was marginally stronger separation between the soil seed bank communities of CP and NF in the dry season (T = −12.7, A = 0.05, P < 0.001) than in the wet season (T = −10.4, A = 0.03, P < 0.001), as a more negative value of T describes a stronger separation between groups. Analysis by MRPP for data pooled across seasons also indicated a strong separation in species composition of the soil seed banks of NF and CP (T = −15.5, A = 0.03, P < 0.001).
3.4. Affinity between Soil Seed Bank and the Standing Tree Community
Only 23% of the tree species (7 out of 30) in the standing vegetation ≥ 5 cm DBH in the CP (mean ± SE species richness plot−1: 4.75 ± 0.24) were represented in the soil seed bank (4.35 ± 0.19), and in NF only 9% of the tree species (1 out of 11) of the standing vegetation ≥ 5 cm DBH (4.50 ± 0.33) was represented in the seed bank (3.00 ± 0.42) (c2 = 1.62, df =2, P = 0.44, Table A1, Table A3 and Table A4). Values of Sørenson’s similarity index (Cs), comparing the species composition of soil seed banks with that of the standing tree (≥5 cm DBH) communities, were higher for CP than NF in both dry and wet seasons, and in the data pooled across both seasons (Table 1). However, values of Sørensen’s index were relatively low (≤0.32) in all cases.
![]()
Figure 6. Non-metric multidimensional scaling ordination diagram of species scores and environmental variables. The environmental variables associated with variation in tree species composition was canopy openness (Cop).
Species codes: ACPE = Acronychia pedunculata; AELA = Aerva lanata; AGCO = Ageratum conyzoides; AGRI = Ageratina riparia; AMOL = Amaranthus oleraceus; AUIN = Austroeupatorium inulifolium; BIPR = Biophytum proliferum; BRMI = Brachiaria miliformis; CETI = Celtis timorensis; COIN = Commelina indhiscens; CRCR = Crassocephalum crepidioides; CYCR = Cyanotis cristata; CYCE = Cynotis ceylanica; CYCO = Cyperus compressus; CYIR = Cyperus iria; CYRO = Cyperus rotundus; DELO = Debregeasia longifolia; DEHE = Desmodium heterophyllum; DESP = Desmodium sp.; DIAD = Digitaria adscendens; ELSC = Elephantopus scaber; ELCA = Elettaria cardamomum; ERPI = Eragrostis pilosa; ERUN = Eragrostis unioloides; EUTH = Euphorbia thymifolia; FIHI = Ficus hispida; GLCO = Glochidion coriaceum; HENI = Hedyotis nitida; HYJA = Hydrocotyle javanica; ILKN = Ilex knucklesensis; ISKU = Isachne kunthiana; KYBR = Kyllinga brevifolia; LESP = Leucaena sp.; LEBI = Leucas biflora; LOTR = Lobelia trigona; LUDE = Ludwigia decurrens; MAIN = Macaranga indica; MAPE = Macaranga peltata; MAESA = Maesa indica; MEMA = Melastoma malabathricum; MISC = Mikania scandens; MIIN = Mimosa sp.; NEMO = Neanotis monosperma; OLCA = Oldenlandia carymbosa; OSRU = Osbeckia rubicunda; OXDE = Oxalis debilis; PHDE = Phyllanthus debilis; PIMI = Pilea microphylla; PLNI = Plectranthus nigrescens; POTR = Pouzolzia triandra; RADE = Rauvolfia densiflora; STIN = Stachytarpheta indica; STVE = Stemodia verticillata; SYCO = Symplocos cochinchinensis; TOCY = Torenia cyanea; UN1. = Unknown spp.; and VIPI = Viola pilosa.
3.5. Non-Native Plant Species in Soil Seed Banks
The non-native species Austroeupatorium inulifolium (Kunth) R. M. King and H. Rob., Ageratina riparia (Regal) R. M. King and H. Rob., Elettaria cardamomum var. cardamomum and Leucaena sp. species constituted 0.4%, 1.4%, 1.1% and <0.1% (i.e. 36, 124, 99 and 3 emergents from the total of 8906) of all emergents respectively in the CP; and 0.7%, 0.6%, 0.0% and 0.3% (5, 4, 0, 2 emergents of the total 714), of all emergents in soil from NF, respectively (Table 3). The density and relative abundance (%) of A. inulifolium and Leucaena sp. seeds were not different between CP and NF, or between seasons. The density and relative abundance of A. riparia and E. cardamomum seeds were higher in soil from the CP than in that from the NF in both dry and wet seasons. Overall, the relative abundance and density of the seeds of all non-native species (pooled data) were higher in the soils of CP than NF (Table 3).
4. Discussion
The cultivation of perennial crops beneath the canopies of tropical forests is a long-standing practice, and the extent of forests affected is increasing as landscapes become more intensively used across all tropical regions. In some cases, these crops will become subsequently abandoned as at our study site in Sri Lanka, although this is rarely quantified. For cardamom, there are published records of plantations in natural forests in India, Lao, Cambodia, Vietnam, Thailand, Costa Rica, Guatemala, El Salvador and Tanzania. Other crops that have analogous management regimes include Brazil nut (Bertholletia excelsa Humb. and Bonpl.), coffee (Coffea spp.), Benzoin (Styrax paralleloneurum Perkins), rubber (Hevea brasiliensis (Willd. ex Adr de Juss.) Muell. et Arg.), cinnamon (Cinnamomum verum Presl.) and cocoa (Theobroma cacao L.). The total area of these crops under cultivation across the tropics is likely to be substantial and increasing, therefore this study is representative of an increasingly important disturbance regime globally.
Cultivation of cardamom in the understorey of a Sri Lankan lower montane rain forest increased the density and changed the composition and diversity of
![]()
Table 3. Relative abundance (%) and mean density (±SE) per sample of non-native species and their statistical significance in the soil seed banks of cardamom plantation (CP) and natural forest (NF) during dry and wet seasons, and in data pooled across both seasons. The statistical significance is evaluated using P value, considering value < 0.05 as significant.
seeds in the soil seed bank. The management adopted for cardamom cultivation increased the abundance of seeds of forbs, grasses, some light demanding tree and shrub species, and non-native species, and these effects persisted even 25 years after active management of the cardamom plantation had ceased. These results are consistent with other studies showing that frequent and/or intense disturbance to forests increases the density and alters the species composition of buried seeds ( Leckie et al., 1999 ; Quintana-Ascencio et al., 1996 ; Sousa et al., 2017 ), although no other studies have demonstrated this phenomenon for forests disturbed by cultivation of understorey perennial crops such as cardamom or ginger.
A direct comparison of soil seed bank densities across studies is difficult because of the different sampling depths used in different studies and the inclusion of surface litter in the samples of many studies (see Garwood, 1989 ). However, the density of seeds in the soil of the abandoned cardamom plantations sampled in Sri Lanka is comparable with that in other disturbed or secondary tropical forests where seeds of weedy species are predominant. For example, Quintana- Ascencio et al. (1996) reported a seed bank density of 4000 seeds m−2 for disturbed tropical rain forest in Mexico, while Butler & Chazdon (1998) found 4535 seeds m−2 in soils of secondary tropical wet forest in Costa Rica. The density of seeds in the soil of relatively undisturbed natural forest at our study site (2231 ± 184 seeds m−2) falls within the range reported for mature tropical forests in other studies, such as the values of 720 - 2341 seeds m−2 for lower montane forest ( Williams-Linera, 1993 ) and 873 - 3632 seeds m−2 for tropical cloud forest ( Alvarez-Aquino et al., 2005 ) in Mexico.
The higher density of seeds in the soil of the abandoned cardamom plantations (CP) than natural forest (NF) at our study site is likely to be associated with an increase in the canopy openness of the CP and a consequent rise in the abundance of forbs and grasses in the ground layer vegetation. Studies elsewhere have also documented a positive response of herbaceous plant abundance to an increase in resource availability, particularly light, resulting from canopy opening (e.g., Lindgren et al., 2006 ; Thomas et al., 1999 ).
Allochthonous seed rain from adjacent pastures and cultivated areas influence the soil seed banks of tropical forest fragments ( Dalling & Denslow, 1998 ; Dupuy & Chazdon, 1998 ; Quintana-Ascencio et al., 1996 ; Young et al., 1987 ). Our study sites are located within 1 km of extensive grasslands established on abandoned tea plantations, and within 600 m of a road. Therefore, it is possible that the density of grasses and forbs has been elevated in both forest types compared to more remote sites. Hence the dispersal of seeds by animals and wind from sources in adjacent agricultural land, as well as reproduction of plants already established within the forest both increase the density of soil seed bank. However, our data suggest that any influence of these factors was not sufficient to obscure the effect of cardamom cultivation. In addition to an increase in the abundance of herbaceous plants, higher inputs of the seeds of light-demanding trees such as Macaranga indica and non-native species such as Ageratina riparia have contributed to the higher density of seeds in the soil seed bank of the CP. These higher inputs result from an increase in the relative abundance of adult individuals of herbs, light-demanding trees and non-native shrubs in the plant community comprising the cardamom plantation ( Dhakal, 2011 ).
Trees represented only 18% and 15% of all seeds in the soil of cardamom plantations and natural forests respectively. Undisturbed forests tend to have a higher proportion of tree seeds in the seed bank than disturbed forests ( Garwood, 1989 ; Leckie et al., 1999 ). As in studies elsewhere ( Kennedy & Swaine, 1992 ; Rico-Gray & Garcia-Franco, 1992 ), seeds of late-successional tree species were almost entirely absent from the soil seed banks of either CP or NF, which can be attributed to seed predation, their tendency to germinate quickly, and their lack of seed dormancy ( Dupuy & Chazdon, 1998 ; Hall & Swaine, 1980 ; Hopkins & Graham, 1983 ; Leckie et al., 1999 ; Miller, 1999). In addition, many tropical tree species reproduce at irregular supra-annual intervals ( Ashton et al., 1988 ; Connell & Green, 2000 ; Hart, 1995 ; Metz et al., 2008 ) and a lack of synchrony between fruit production of late-successional species and the time when the seed bank was sampled would lead to under-representation of these species in the sample. This combination of factors might explain the low correspondence in the composition of tree species in the soil seed banks and the standing tree community in both CP and NF (Table 1), which is consistent with studies of both disturbed and undisturbed tropical forests ( Dalling & Denslow, 1998 ; Dupuy & Chazdon, 1998 ; Roberts, 1981 ; Tekle & Bekele, 2000 ).
Cardamom plantations had a greater similarity in the species composition of trees in the soil seed bank and the standing tree community than natural forests, which is partially explained by the representation of light-demanding species in both above- and below-ground communities in CP but not NF. For example, the seeds of early-successional light-demanding species such as Macaranga peltata and M. indica were present in the soils of abandoned cardamom plantations, but absent from the seed banks of adjacent natural forests. This result reflects the much greater abundance of established trees of these species in the CP ( Dhakal et al., 2012 ). Other studies of montane forest have reported an increased emergence of pioneer species after soil-surface disturbance from clearance of understorey by humans ( Williams-Linera et al., 2016 ; Osumi & Sakurai, 1997 ; Williams-Linera, 1990 ).
Seeds of the non-native species A. riparia and Elettaria cardamomum were present at higher densities in the soil of cardamom plantation than natural forest. The greater abundance of the Elettaria presumably reflects increasing pro- pagule pressure arising from reproduction of the residual cardamom plants, while a higher density of A. riparia suggests that disturbance associated with cardamom cultivation may increase the likelihood of non-native species invading the forest community. This finding supports the hypothesis that disturbance increases the risk of colonisation by non-native invasive species ( Dalling & Denslow, 1998 ; Lin et al., 2006 ; Tang et al., 2006 ). Taken together, the higher abundance of herbs, light-demanding trees and non-native species in the seed banks of CP than NF explains their separation into distinct clusters in ordination space, and suggests that they represent distinct floristic communities. This distinction is not explained by variation in altitude among plots, but is associated with differences in canopy openness of plots between the two forest types.
The pattern of species richness and diversity of the soil seed banks of cardamom plantations and natural forest were not consistent between seasons. Soil seed banks had higher species richness and diversity in abandoned cardamom plantations than natural forests during the dry season, but these contrasts were reversed in the wet season. Some studies carried out in disturbed tropical forests have found that the addition of pioneer species to the tree community can increase the species diversity and richness of the soil seed bank ( Arevalo & Fernandez-Palacios, 2000 ; Quintana-Ascencio et al., 1996 ), but in our study system the number of light-demanding species added to the tree community is not sufficient to offset the reduction in species richness associated with disturbance. This comparison is limited by the relatively low number of samples taken from NF, which resulted in the species accumulation curves failing to reach an asymptote. Therefore, it remains possible that the relative difference in species richness between forest types for larger sample sizes would be lower than recorded by us, or even reversed.
5. Conclusion and Implications for Conservation
Cardamom cultivation affected the density and community structure of soil seed banks in a tropical lower montane forest in Sri Lanka. The higher density and/or percentage of the seeds of grasses and forbs in cardamom plantations might increase the abundance of seedling germinants representing these life-forms relative to germinants of trees. The low similarity in the composition of the soil seed bank and that of the standing tree community, and the absence of most tree species from the soil seed bank of the abandoned cardamom plantation, implies that the seed bank is an unlikely source of propagules for restoring the tree community to its former condition in the undisturbed forest. Instead, restoration strategies that rely on other sources of propagules, such as protection of newly dispersed seeds or enrichment planting of seedlings, require further investigation in order to define management prescriptions for forest restoration and biodiversity conservation.
Acknowledgements
My special thanks go to Profs David Burslem and Michelle Pinard for their guidance and help throughout this study. I also thank Profs Nimal Gunatilleke and Savitri Gunatilleke for their support in the field. I thank the Darwin Initiative (Project No. 15010) for financial support and the Forest Department of Sri Lanka for granting permission to carry out research in the Knuckles Conservation Area. I am also grateful to Midland State Plantation for allowing us to carry out research in the estates’ cardamom plantations.
Appendices: Supplementary Data
![]()
![]()
![]()
Table A1. Total number of emergents from soil seed bank samples taken from abandoned cardamom plantations (CP) and natural forests (NF) in lower montane forests in Sri Lanka in the dry (August) and wet (February) seasons and for pooled data from these two seasons. Late-successional tree species are indicated by an asterisk.
![]()
![]()
Table A2. Non-metric multidimensional scaling correlations for the soil seed bank species with the first three ordination axes in cardamom plantation and natural forest. Pearson’s parametric (r) correlation with ordination axes, n = 103. Species variable with r ≥ 0.30 are highlighted in bold.
![]()
Table A3. Abundance of overstorey species recorded in the experimental plots in cardamom plantation.
![]()
Table A4. Abundance of overstorey species recorded in the experimental plots in natural forest.