Half-Life of Glyphosate on the Control of Water Hyacinths in Water Tanks ()
1. Introduction
The chemical method, which advocates the use of specific herbicides to control aquatic plants, has been the most widely used in different places in the world due to low cost and especially the speed, easy of application and control efficiency [1] [2] [3] . The world’s most used herbicides to control aquatic macrophytes are: 2,4-D; diquat; endothall, a copper-based compound; fluridone; imazapyr and glyphosate [4] . The production of knowledge in this area in Brazil has been small, given the prohibitive legislation in loco studies [5] . The studies developed in the country, reason of that prohibition, have been performed in controlled environments and in closed systems, with results showing herbicide use efficiency in controlling several species of aquatic plants [6] [7] [8] [9] .
According to [5] , the chemical control of aquatic plants is restricted to a few herbicides due to restrictions imposed by legislation, to the environmental impact, to the market size for the private sector and to the application of technology. The environmental restrictions are the most important factors because the waterways are used for many purposes, such as a source of water for human and animal consumption, crop irrigation, leisure activities, navigation and hydraulic energy generation.
Although the different work done to prove efficiency of the use of various active ingredients in the control of aquatic macrophytes, few environmental impact studies have been conducted. Environmental impact studies have been little exploited to date due to the limitation of the use of chemicals in aquatic environments [10] for the protective intervention of IBAMA (Brazilian Institute of Environment and Renewable Natural Resources). However, much more important than the action of the product in the management of macrophytes is its direct action on integration with the environment in question. Among studies performed, the ones mentioned are those monitoring the effects of the herbicides on the quality of the water and the sediments [11] [12] [13] [14] and more rarely those that have investigated, besides the water quality parameters, the molecule residues in plants and in the water, in the reservoirs or mesocosms [13] [14] [15] .
Thus, an accurate study of the use of herbicides to control the aquatic macrophytes in closed aquatic environment, from the point of view of environmental impact, may systematize data scientifically treated, and form knowledge to evaluate its behavior within the environment. Therefore, it will contribute in the definition of public policies by regulatory agencies in facing the serious problem represented by the uncontrolled proliferation of macrophytes in water bodies, particularly, by obstruction of the turbines in reservoirs for power generation. This fact has a direct effect on the production cost of electrical energy, which is essential for economic, technological and social development of the country.
In the present experiment the herbicide glyphosate was chosen because it is registered in several countries for the control of aquatic macrophytes, being one of the main products studied, used in the world and with great potential in the management of aquatic weeds. Thus, it was chosen to study it in a controlled and closed experimental field, in the management of (Eichhorniacrassipes) in reservoirs. The behavior of the product’s half-life was analyzed, aiming to contribute to the risk analysis formulation of environmental impact of the use of this product to control aquatic weeds.
2. Materials and Methods
The study was developed in the Experimental Area of the Núcleo de Pesquisas Avançadasem Matologia (NUPAM) belonging to the Faculdade de Ciências Agronômicas da UNESP (Department of Agronomic Sciences of the University of São Paulo), Botucatu (SP) campus, during the months from May to November 2011. The local climate, according to the Köppen method, is classified as hu- mid mesotherm (Cfa), presents average temperature of the hottest month above 22˚C and small water deficiency between April and August. Eight (08) fiber-ce- ment tanks were used, four for each treatment with a storage capacity of 250 liters, without water flow and without replacement of the evapotranspired water (“worst case”). The water supplying the tanks came from the Companhia de Saneamento Básico do Estado de São Paulo (SABESP) (Sanitation Utility of the State of São Paulo). The samples were collected in the lower third of the tanks.
2.1. Treatments
The treatments used in the experiment were called: 1―tank without water hyacinths and with application of glyphosate; 2―tanks with water hyacinths and with application of glyphosate.
For the set of conditions of the tanks containing macrophytes, the species analyzed was Eichhorniacrassipes (Mart.) Solms (water hyacinth). The plants were collected from the Tietê River, Botucatu region (SP) and placed in boxes to provide 90% occupancy of the tank surface.
The herbicide control was carried out by application of glyphosate in the recommended maximum dose since the recommendations for the study of environmental impact are to look for the worst scenario “worst case”. According to the former registration of the product, the Rodeo, in Brazil, the recommended dose was 7.0 L∙ha−1 or 3402 g∙ha−1 of acid equivalent. The surfactant Alterbane was added to the herbicide stock at a concentration of 0.5% (466.6 g∙L−1) [16] .
The tanks were sprayed, using the carbon dioxide precision equipment (back- pack sprayer), provided with plywood bar containing two flat jet nozzles Jacto XR 110.02, operating at a pressure of 2 kgf∙cm−2 (20 Kpa), providing an even distribution of the glyphosate in the tank and a solution consumption corresponding to 200 L∙ha−1. The calibration was performed on site based on the applicator speed in regards to the area worked.
2.2. Evaluations
The values obtained in the control efficiency of water hyacinth were submitted to variance analysis and to Tukey test, considering a 5% level of significance. The evaluations of effectiveness were carried out visually after 2, 4, 8, 16, 32 and 64 days after application (DAA), using percentage scale, where 0 (zero) represents no control and 100%, the total plant control [17] .
The water samples to determine the glyphosate residues were collected on the day of the application (day zero) and on 2, 4, 8, 16, 32 and 64 DAA, in the morning, always at the same time, at 11 a.m. The depth of the collection was conducted between 15 - 30 cm below the water level, and the samples were placed in plastic containers of 200 mL and stored in a freezer at −18˚C, in which the residue analyses were subsequently performed.
The concentrations (mg∙Kg−1) of glyphosate and AMPA (main metabolite of glyphosate) residues were determined. For determination of compounds in water, the samples were thawed, stirred, and filtered directly on Millex HV filter (Millipore) 0.45 μm, provided with a 13 mm durapore membrane, and placed in a 9 mm amber-colored vial (Flow Supply), with a capacity of 2 mL, for further quantification by LC-MS/MS.
The molecular mass and fragments generated from each molecule are shown in Table 1. The fragment used for quantification of each of the compounds was always the first presented in Table 1, for each compound. The analytical curves for the compounds were constructed in the concentration ranges described in Table 2. The linear model used in the equations was quite adequate.
The method used was by high performance liquid chromatography (HPLC) and mass spectrometer with a mass selective detector, presenting detection limit of compounds at 0.001 mg∙kg−1 of the glyphosate.
Before each collection, the volumes of the tanks were measured so that, later, the load (mass) of glyphosate could be calculated in time. The measurement was made with a ruler, considering the relationship between the height/volume of the initial mark of each tank and the height/volume of the mark corresponding to the level of the water at the time of the collection.
The mathematical model of decay was applied to describe the decrease in the concentration of glyphosate and loads in water and to determine the half-life of the compounds.
![]()
Table 1. Molecular mass and secondary ions of the analyzed compounds.
![]()
Table 2. Analytical curves and concentration ranges for each compound analyzed.
In this experiment two tanks, repetition 1 (No 1) of the treatment plant with glyphosate application and repetition 1 (No 5) without treatment plant application of glyphosate, had to be discarded. The reason was the failure to identify residues in the 1st analysis, immediately after the application, to the tank N˚ 1 and small concentration to the tank N˚ 5; in the following analyses of this tank, no residues were detected. Since the calibration was carried out with water before application, a volume was certainly left in the system, between the bar and the spray nozzles. Moreover, as these tanks were the first to receive the herbicide application and since they were set in sequence in the experimental field, it is easy to understand why no residues were found.
3. Results and Discussion
The data obtained in the present experiment with the use of the herbicide glyphosate, in the evaluated dose, showed a percentage of average control of 91.3%, presenting excellent efficiency in the control of the water hyacinths, agreeing with previous works [6] [7] [18] .
The values of the glyphosate residues (concentrations and mass) and tank volumes found from dates 0, 2, 4, 8, 16, 32 and 64 DAA, for both sets of tanks, are presented in Table 3.
![]()
Table 3. Values of glyphosate residues (concentration and mass) and tank volumes found from dates 0, 2, 4, 8, 16, 32 and 64 Days after Application (DAA).
For those tanks which have not received plants, it can be verified that there was a reduction of loads in 99.5%, 90.9% and 99.3%, in boxes 2, 3 and 4, respectively. As for the tanks colonized by the water hyacinth, the decay in the boxes 2, 3 and 4 represented, respectively, 97.1%, 96.1% and 96.6%.
Likewise the concentration, the average load of glyphosate found in the tanks was greater, sooner after the application, being four times greater where there was no presence of water hyacinth.
However, over time, the mass found in the reservoirs without plants matched the one found in reservoirs containing plants. From 32 DAA onward, the loads found in the tanks without the hyacinth were lower compared to the tank containing plants. The results show that from the metabolism of glyphosate and subsequent control effectiveness or death of the plants onward, the process of decomposition of dead plants released the product into the water in the tanks keeping its content, even if low, but higher thanks that received direct application of the product.
To calculate the AMPA mass in the water in the treatments with and without water hyacinths, the water volumes were used in the tanks in each moment of sample collections and the respective concentrations are presented in Table 4. In the analysis of AMPA parameter, it was expected to see the same behavior in the mass obtained for the glyphosate. However, as can be seen, the larger masses found in the treatment that received the direct application of the product without the presence of water hyacinth, lasted throughout the study period. This behavior suggests that the metabolism of AMPA through the bioaccumulation pro- cess is superior to glyphosate. It was not possible to compare results of glyphosate and AMPA loads with other studies, since these are lacking in the available literature.
In order to adjust the data observed of glyphosate residues in water over time, it was used a mathematical model described by Equation (1):
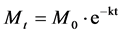 (1)
(1)
where: Mt―glyphosate mass at moment t; M0―glyphosate mass at the initial time considered; k―decay coefficient and t―time.
Applying the Naperian logarithm in the Equation (1), it is obtained:
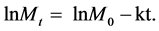 (2)
(2)
This equation represents a linear model where the coefficient (K) identifies the mass decay of glyphosate over time. Since three tanks were used in the experiment, the average load was calculated, at times considered of the samples, to determine the average decay value for glyphosate. In order to obtain this value, the logarithm (Ln) was applied to mass values observed (Figure 1) getting the decay value for the tanks without plants of 0.115 day−1.
From the value found in the coefficient of average load decay of glyphosate, average values of glyphosate were simulated using the mathematical model of Equation (1) (Figure 2). With this model, it was possible to estimate the half-life of glyphosate applied directly to the water, without the presence of water hyacinths. Considering the estimated average load, the half-life was six days.
![]()
Figure 1. Logarithm of the observed values of average mass (mg) of glyphosate in the tanks with hyacinth and with application of glyphosate.
![]()
Table 4. Values of AMPA mass (mg) in the water in the treatments, in each tank, in the treatments with and without water hyacinth from dates 0, 2, 4, 8, 16, 32 and 64 Days after Application (DAA).
![]()
Figure 2. Observed and simulated values of average mass (mg) of glyphosate in the tanks with water hyacinth. Back symbols are values of average mass. Bars are values of average mass maximum and minimum. Dashed lines are simulated values of average mass.
Likewise, the simulation was performed for the tanks receiving the colonization of water hyacinth. The value of the coefficient K and the observed and simulated values of the average load are shown in Figure 3 and Figure 4, respectively. For the conditions of the colonized tanks by water hyacinth, the decay value was calculated equal to 0.043 day−1. From Equation (2), it was estimated the glyphosate half-life in water for the treatment with the macrophyte, resulting value of 16 days.
The simulated values for the half-life of glyphosate in water, in both situations, are in accordance to [19] [20] [21] [22] which reports that the average life of the molecule in water can range from 1 to 51 days and to [23] , which states that, depending on the conditions of the water body, particularly to those linked to the full microbe activity can vary between a few days to two weeks.
Studies performed in a forest ecosystem [24] [25] have shown that glyphosate had quickly dissipated in the lake waters with many suspended sediments, with the half-life ranging from 1.5 and 11.2 days.
According to FPPD 2012 [26] , Mackay et al. 2006 [27] and Gassemini et al. 1981 [28] , cited by Mercurio et al. 2014 [29] , the half-life of glyphosate in fresh water was estimated between 28 and 87 days.
Furthermore, in the study developed by [30] , which was a river water body (open system), it was observed that the half-life of glyphosate in this environment had varied between 60 and 100 hours.
[31] evaluated the environmental fate of glyphosate in the water-sediment system with focus on its microbial metabolization. The results found demonstrated the key role of sediments in its degradation. Also, Glyphosate was detected below detection limit in the water compartment at forty days.
In compliance with [32] , this is a low toxicity herbicide that quickly dissipates in the environment. [33] compliments reporting that low bioavailability of glyphosate in natural waters is due to its quick degradation and quick microbial decomposition.
![]()
Figure 3. Logarithm of the observed values of average mass (mg) of glyphosate in the tanks without water hyacinth and with application of glyphosate.
![]()
Figure 4. Observed and simulated values of average mass (mg) of glyphosate in the tanks without water hyacinth. Back symbols are values of average mass. Bars are values of average mass maximum and minimum. Dashed lines are simulated values of average mass.
4. Conclusions
The study has shown that glyphosate applied directly to the water surface or to controlling water hyacinth plants in closed aquatic environments is rapidly degraded and has no residues which would preclude its use. The average life of glyphosate in water for the control of macrophytes was estimated at 16 days and the decay calculated value was equal to 0.043 day−1.
Therefore, the use of glyphosate in the control of aquatic plants can be recommended for use in continuous flow aquatic environments, since it has more favorable characteristics to dissipate and degrade herbicides, due to being less drastic than the one evaluated in this work.
Acknowledgements
We thank the School of Agricultural Engineering―University of Campinas and the Weed Science Center (NUPAM) of the College of Agricultural Sciences São Paulo State University for generous support in this project.