Modification of Caster Oil and Study Its Efficiency as Corrosion Inhibitors in Formation Water Media ()
1. Introduction
Corrosion is the destructive attack of a material by reaction with its Environment [1] . Corrosion is a chemical or electrochemical oxidation process, in which the metal transfers electrons to the environment and undergoes a valance change from zero to a positive value [2] . The serious consequences of the corrosion process have become a problem of worldwide significance [3] . Corrosion control is achieved by recognizing and understanding corrosion mechanisms, using corrosion inhibitor materials. A corrosion inhibitor is a chemical substance that, when added in small concentration to an environment, effectively decreases the corrosion rate [4] - [9] .
The use of chemical inhibitors to decrease the rate of corrosion processes is quite varied. In the oil extraction and processing industries, inhibitors have always been considered to be the first line of defense against corrosion. A great number of scientific studies have been devoted to the subject of corrosion inhibitors. However, most of what is known has grown from trial and error experiments, both in the laboratories and in the field. Rules, equations, and theories to guide inhibitor development or use are very limited. Inhibitors have been classified by their chemical functionality into: inorganic inhibitors, organic inhibitors [10] - [16] .
Organic inhibitor are applied extensively to protect metals from corrosion in many aggressive acidic media (e.g., in the acid pickling and cleaning process of metals). Different kinds of organic compounds are used as corrosion inhibitors for iron alloys in various acid media such as aromatic compounds with positively charged amine groups, Sodium sulfonates, phosphonates, or mercaptobenzotriazole (MBT) are used commonly in cooling waters and antifreeze solutions [17] [18] [19] [20] . In the present study used ammonium sulfonated castor oil (ASC) to produced water soluble amino compound as corrosion inhibitor.
2. Experimental Techniques
2.1. Material and Instrument
Caster oil, sulfuric acid, ammonia solution, mechanical stirring, bath, evaporation rotary, viscometer, IR spectra, RP 6000, carbon steel (C1010) standard strips and potentiostat.
2.2. Experimental Procedure
2.2.1. Ammonium Sulfonated Castor Oil (ASC) Preparation
In a 500 ml three-neck round bottom flask, 250 ml of castor oil and 75 ml of sulfuric acid were charged and the whole were stirred for 24 h at room temperature. At the end of reaction, the reaction was cooled to room temperature and then, ethyl acetate (50 mL) was added to the reaction mixture with stirrer for 30 min, followed by filtration to separate the precipitate from the mixture medium. The precipitate obtained was purified by column chromatography (silicagel, petroleum ether/ethyl acetate (100/1, v/v) as eluent) to give sulfonated castor oil (SC). Then the (SC) dissolved in distilled water and neutralized with ammonium solution to give ammonium sulfonated castor oil (ASC) [21] [22] [23] [24] .
2.2.2. Preparing the Metal Specimen for Test
A standard strip of carbon steel alloy (C1010) is shown in Figure 1 with dimensions of 3 cm long, 1.24 cm wide and 0.14 cm thick. The total area of immersed strip is 8.45 cm2. Carbon steel strips were used to evaluate the prepared compounds as corrosion inhibitors. The faces of each specimen were grinded and polished
by emery cloth paper to 400 micron. Then specimens degreased with acetone, and are washed with distilled water and ethanol. Hot air were used to dry the sample and stored in a desiccators containing silica gel during the period in between polishing and Tafel measurements. The composition of these alloys was illustrated in Table 1.
2.2.3. Preparing the Corrosive Environments
In present study, we use formation water from Iraqi southern oil Company as corrosive environment. The compositional analyses of formation waters carried out in south oil company―Basrah, as shown in Table 2 and Table 3.
3. Result and Discussion
3.1. Characterization of the Ammonium Sulfonated Castor Oil
The new compounds were characterized, by FTIR Spectroscopy―technique, as a viscous solution by using sodium chloride disc. Spectra [25] [26] are shown in Figure 2 and Figure 3. From IR Spectrum, the SO3 group and N-H group don’t give absorption in Figure 2, but they gave absorption in Figure 3 as showed in Table 4.
3.2. Evaluation of Ammonium Sulfonated Castor Oil as Corrosion Inhibiter
A potentiostat was used to measure current density vs electric potential in order to calculate the corrosion rates. The electrochemical results obtained from polarization experiments through “Tafel plots” performed in corrosive environment (formation water) in the absence and presence of different specified concentration of (ASC) (from 5 ppm to 30 ppm) are showed in Figure 4, Figure 5 and Table 5 at constant temperature 26˚C. The Corrosion rate (CR) was measured in (mm/y) units, and to convert corrosion rate (CR) from (mmy) to (mpy), Equation (1) were used [27] :
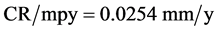 (1)
(1)
![]()
Table 1. Chemical composition of carbon steel alloy.
![]()
Table 2. Analysis for the formation water.
![]()
Table 3. Ions concentration (ppm) in formation water.
![]()
Figure 3. IR spectra of ammonium sulfonated caster oil (ASC).
![]()
Table 4. Explain IR spectra for both caster oil and ammonium sulfonated caster oil.
Tafel plots employed for rapid evaluation inhibitors in order to determine their effectiveness on the corrosion rates of carbon steel. From Table 5 and Figure 5, the prepared compound (ASC) showed high inhibition efficiency for corrosion
![]()
Figure 4. Tafel plot of formation water without inhibitor.
![]()
Figure 5. Tafel plot with optimum value 25 ppm of inhibiter (ASC).
![]()
Table 5. Corrosion inhibiter for compound (ASC) at constant temperatur (25˚C).
rate, Ecorr and Icorr of carbon steel in formation water after added various concentrations. From these results, the corrosion rate reduced as concentration increases. The compound (ASC), Table 5 showed the optimum inhibition concentration is 25 ppm with efficiency 98% whereas at lower (A) concentration a decrease in efficiency can be observed due to its chemical structure, in 5 - 20 ppm has lower solubility and that leads to a less adsorption on metal surface leads to increasing Icorr then reduce its efficiency. In high concentration (30 ppm) the increasing the molecules of (ASC) which have a large chain that caused a steric hindrance on metal surface leads to a difficulty in its adsorption process leads to increasing Icorr then reduce its inhibition efficiency(26). On the other hand, the βc of compound (ASC) Table 5 is larger than βc of Blank this shows that compound (ASC) behaves as cathodic inhibitor. The efficiency of an inhibitor can be expressed by the measure of this improvement according to the following equation [28] [29] .
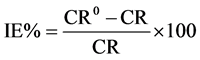 (2)
(2)
3.3. Effect Increasing Temperature on Corrosion Rate
The effect of temperature on inhibition efficiency of the inhibitors on carbon steel (CS) alloy can be studied depending on two principles, the first principle includes increasing the efficiency as it decreases in temperature; this due to physical adsorption mode for the inhibitor on the surface of metal or alloy, and the second principle includes increasing the efficiency as temperature increases; it results from chemical adsorption mode for the inhibitor on the surface of metal or alloy [30] [31] [32] .
The present study of the effect of temperature on inhibitor properties and corrosion inhibitor for CS and alloy was studied at range (30˚C - 50˚C) as is shown in Table 6.
This table showed that this inhibitor have some effects on both, the cathodic and anodic processes. This indicates a modification of the mechanism of cathodic hydrogen evolution as well as anodic dissolution of iron, which suggests that the above inhibitors can be used to inhibit the corrosion process of CS and their suppression of cathodic process by the covering of CS surface with monolayer is due to the adsorbent of inhibitors molecules. It can also be the anodic Tafel constant (βa) slopes which has variable values between increasing and decreasing values whereas the decreasing may be described to the changes in charge transfer
![]()
Table 6. Effect temperature on corrosion rate of compound (ASC) at optimum value (25 ppm).
resistance. From Table 6, the compound (ASC) showed that the increasing corrosion rate with increasing the temperature as well as increasing in Icorr lead to decreasing Rct and decreasing inhibition efficiency and decreasing in both βa and βc with increasing temperature refers to increasing in both anodic and cathodic reactions respectively. The decreasing in inhibition efficiency as temperature decreased can be interpreted due to that desorption is aided by increasing the corrosion. Decreasing in efficiency as temperature increased also results from the decreasing in viscosity of the inhibitor solution then increasing the diffusion of ions in solutions and the decreasing the stability of protective film on metal surface that leads to decrease the activity of the inhibitor. Increasing temperature also increases the diffusion of ions in solutions and decrease the stability of protective film on metal surface which leads to decrease activity of the inhibitor. But at 35.40˚C, the inhibition efficiency increasing as increasing temperature due to the adsorbed process via chemical mode due increasing efficiency with increasing temperature, the shifting in Ecorr value toward positive direction [33] [34] [35] .
4. Conclusion
The inhibition characteristics of compound (ASC) were studied for iron corrosion in formation water. Electrochemical (Tafel polarisation and EFM) method was applied. Polarisation measurements showed that the compound (ASC) acted as cathodic inhibitor. The surfactants chemisorbed on the electrode surface without modifying the mechanism of anodic and cathodic reactions. The cathodic process is activation controlled even in the presence of surfactant. The inhibition efficiency of the surfactants generally increases with the increasing in surfactant concentration. From these results the ammonium sulfonated castor oil (ASC) can be used as anti-corrosion inhibitor for pipeline of crude oil.