The Effect of Nitrogen and Phosphorus Ratios and Electrical Conductivity on Plant Growth ()
Received 11 May 2016; accepted 16 August 2016; published 19 August 2016

1. Introduction
The use of plant growth retardants (PGRs) is becoming increasingly restricted due to environmental concerns surrounding their use. Non-chemical alternatives to PGRs, such as temperature management [1] [2] , light quality and intensity [3] [4] , mechanical stress [5] , and humidity stress [6] can be labor intensive and expensive. One under evaluated potential alternative to PGR use is macronutrient management. Kavanova [7] found that supplying Lolium perenne with low phosphorous (P) levels resulted in a decrease in leaf elongation, which was attributed to a decrease in both cell division rate and cell length. Rideout [8] found similar results with tomato (Lycopersicon esculentum Mill.) seedlings.
Growers generally believe that ammonium (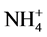 ) may promote stem elongation compared to nitrate (
) may promote stem elongation compared to nitrate (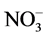 ). However, González-García [9] showed an opposite trend in chives (Allium schoenoprasum L.), and no effect in basil (Ocimum basilicum L.). Nelson [10] was in agreement with González-García for impatiens (Impatiens wallerana), but showed no effect for petunias (Petunia × hybrida), and showed opposite results for tomatoes and marigolds (Tagetes erecta).
). However, González-García [9] showed an opposite trend in chives (Allium schoenoprasum L.), and no effect in basil (Ocimum basilicum L.). Nelson [10] was in agreement with González-García for impatiens (Impatiens wallerana), but showed no effect for petunias (Petunia × hybrida), and showed opposite results for tomatoes and marigolds (Tagetes erecta).
Growth suppression may occur when ion concentrations increase to a point where osmotic stress, due to high electrical conductivity (EC), is induced and water availability becomes limited [11] [12] . Judd [13] found that higher fertilizer concentrations suppressed growth (plant height + width) of impatiens.
As nutrient concentrations for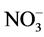 ,
, 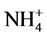 and P also affect the EC levels, it was decided to further examine this topic. The hypothesis of this study was that modified
and P also affect the EC levels, it was decided to further examine this topic. The hypothesis of this study was that modified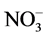 ,
, 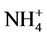 and P ratios can be used as a viable means of plant height control. This study evaluated the effects of several ratios (
and P ratios can be used as a viable means of plant height control. This study evaluated the effects of several ratios (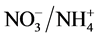 ,
,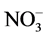 /P,
/P, 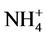 /P) on plant height. The first objective of this study was to quantify the effects of
/P) on plant height. The first objective of this study was to quantify the effects of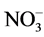 ,
, ![]() and P ratios on plant height using the same total ionic strengths in solution (EC). The second objective was to evaluate the effects of these same ratios at four ECs to help establish any potential links between EC, nutrient ratio and plant height. This study was conducted in aerated nutrient solutions using 3 different species.
and P ratios on plant height using the same total ionic strengths in solution (EC). The second objective was to evaluate the effects of these same ratios at four ECs to help establish any potential links between EC, nutrient ratio and plant height. This study was conducted in aerated nutrient solutions using 3 different species.
2. Materials and Methods
2.1. Treatment Formulation
Control treatments were based on a 1/2-strength modified Hoagland’s solution #2 [14] with an EC of approximately 1.2 dS・m−1 (Table 1). Treatment formulations were created by adjusting ionic ratios (mM/mM). To compensate for alterations in the anion/cation balance and EC, slight modifications were made to the concentrations of other ions so that all formulations provided 10 meq・L−1 anions and 10 meq・L−1 cations in order to obtain a similar EC.
In the first objective, ![]() /
/![]() ratios were modified in a set of 7 different ratios while total N remained constant. To compensate for the increase in
ratios were modified in a set of 7 different ratios while total N remained constant. To compensate for the increase in ![]() levels, K+, Ca2+, and
levels, K+, Ca2+, and ![]() concentrations were adjusted so that K+/Ca2+ ratios remained the same.
concentrations were adjusted so that K+/Ca2+ ratios remained the same. ![]() /P ratios were modified with either
/P ratios were modified with either ![]() being constant, or P being constant (Table 1).
being constant, or P being constant (Table 1).
Similarly, ![]() /P ratios were modified with either a constant
/P ratios were modified with either a constant ![]() or a constant P concentration. In the second objective, ratios from objective #1 were repeated at EC’s of 0.6, 1.2, 2.2 and 4.0 dS・m−1. The following ratios were used (mM/mM): 10, 15, 20, 30 and 60 for
or a constant P concentration. In the second objective, ratios from objective #1 were repeated at EC’s of 0.6, 1.2, 2.2 and 4.0 dS・m−1. The following ratios were used (mM/mM): 10, 15, 20, 30 and 60 for ![]() /P; 1, 1.5, 2.0, 3.0 and 4.0 for
/P; 1, 1.5, 2.0, 3.0 and 4.0 for ![]() /P and 3.25, 4.7, 7.5, 16.0 and ∞ for
/P and 3.25, 4.7, 7.5, 16.0 and ∞ for ![]() /
/![]() .
.
2.2. Treatment Creation and Application
Treatments were provided by dissolving potassium nitrate (KNO3), calcium nitrate [5Ca(NO3)2∙NH4NO3∙18H2O], ammonium nitrate (NH4NO3), magnesium nitrate [Mg(NO3)2・6H2O], monopotassium phosphate (KH2PO4), potassium sulphate (K2SO4) and magnesium sulphate [MgSO4∙7H2O] into 100 L deionized (DI) H2O in 100 L sealed plastic bins. The bins were cleaned and sterilized thoroughly between the different experiments to prevent any alteration in the nutrient solution due to microbial activity. One gram of micronutrient mix (Plant Prod®, Bramalea, ON)/100L was added to each solution. When solutes had completely dissolved, samples were sent to Agri-Food Laboratories (Guelph, Ont., Canada) to check for accuracy.
Treatment application involved filling 1-L ceramic pots, 12 cm in diameter, with a nutrient solution from the marked 100-L bins. Pots were topped up as needed and were flushed out and refilled every week. Treatment samples were taken prior to weekly flushes for the measurements of EC (Hanna® Instruments HI 8733, Ann Arbor, Michigan) and pH (pH18 Aqualytic®, Dortmund, North Rhine-Westphalia, Germany) levels.
2.3. Plant Material
Tomato (Solanum lycopersicum Mill., cv. Roma) and marigold (Tagetes erecta L., cv. Vanilla) seeds were sown
aModified 1/2 strength Hoagland’s solution served as a control.
in 200-cell rockwool plug trays that had been soaked in DI water, and then covered with a thin layer of fine- grained vermiculite. Sunflowers (Helianthus annuus L., cv. Sunrich Orange) were also used in the second objective, but were sown in standard plastic 200-cell plug trays containing Sunshine® Professional Growing Mix LP5 (SunGro Horticulture Canada Ltd., BC, Canada), covered with fine-grained vermiculite, and germinated in a plastic misting chamber inside a glass greenhouse. The reason for using a growing mix was due to the lack of germination in the rockwool plugs. After germination, seedlings were hardened off gradually over a 1 week period before transplantation into a hydroponic system. Planting was done twice for each crop.
2.4. Transplanting into Hydroponics
Seedlings were transplanted into a hydroponic system roughly 11 days after initial seeding for sunflowers and 22 days after seeding for both marigolds and tomatoes. Transplanting involved inserting roots, still attached to either rockwool or growing mix, into a tapered hole of roughly 3.5 cm in diameter at the center of a styrofoam disk. Disks were approximately 10.5 cm in diameter and 2.5 cm thick. Each disk was stabilized on top of a pot with a piece of tubing.
2.5. Bench Setup
Pots were spaced roughly 20 cm apart center to center and were arranged with six plants per row across the bench. Benches were divided into 2 equal halves, each made up of a complete set of treatments and each row of 3 plants was allocated to one treatment (experimental unit). To aerate the solutions, compressed air was provided, and regulated to 500 - 1000 kPa. Air was distributed to each pot through additional networks of 3 air lines, which ran between 2 rows. Each pot was aerated by a smaller tube, which was placed into each pot 4 days after transplanting to aerate the solutions.
2.6. Greenhouse Setup
All experiments were conducted in a 100 m2 glass greenhouse at the University of Guelph, Guelph, Ont., Canada, from April 2010 to November 2012. The greenhouse had top vents, in addition to cooling with water chillers when temperatures exceeded 22˚C and heated when temperatures dropped below 20˚C. A shade curtain was set to open entirely when outside light levels were less than 500 W・m−2 and to close when outside light levels surpassed 700 W∙m−2. No artificial sources of light or CO2 were used.
2.7. Evaluation
Data were collected on 2 plants per experimental unit when the plants flowered (6 weeks after transplanting), as the plant at the outside of the bench was considered guard plant. Plant height, as well as root and shoot weight were recorded. Shoot dry weight refers to the dry weight of all plant parts above the styrofoam plate. Plants were dried for 3 weeks in a drying room (at 45˚C) before root and shoot dry weights were determined.
2.8. Statistical Analysis
A Type I error rate of α = 0.05 was used in all statistical analyses. The experimental set-up used a randomized complete block design with 2 plants per treatment per block and replicated once over time (season). The results of the 2 plants from each block were averaged before analysis. Data were analyzed using a mixed factorial model (MixedProc of SAS® version 9.3, SAS Institute Inc., Cary, N.C.) with a Tukey’s method for multiple comparisons. The sources of variance accounted for in the first objective were nutrient ratios, blocks, replications, and the interactions between these factors. A 2 × 2 treatment design was used in the second objective, with EC as an added source of variation. Regression analysis was performed between nutrient ratios and dependent variables, and the REG procedure was used to generate an equation of the trend.
3. Results
3.1. ![]()
Marigold height increased linearly with increasing ![]() ratios in Spring 2010 (Table 2) but not in Summer 2010 (data not shown).
ratios in Spring 2010 (Table 2) but not in Summer 2010 (data not shown).
For tomato, ![]() ratios did not affect plant height in Spring (Table 2) or Summer 2010 (data not shown) but did affect shoot weight in Spring 2010 (Table 2), as shoots at a
ratios did not affect plant height in Spring (Table 2) or Summer 2010 (data not shown) but did affect shoot weight in Spring 2010 (Table 2), as shoots at a ![]() ratio of 16 were heavier (+31%) than those at a ratio of 2.4.
ratio of 16 were heavier (+31%) than those at a ratio of 2.4.
![]()
Table 2. Height (cm) and shoot dry weight (g) of marigold and tomato plants at flowering (Spring, 2010) in nutrient solutions with varying ![]() (mM/mM) ratios at an EC of ~1.2 dS∙m−1.
(mM/mM) ratios at an EC of ~1.2 dS∙m−1.
aMeans followed by the same letter(s) within a column are not significantly different at P ≤ 0.05. bControl. cNA= Not available. dStandard error (se) with n = 2. e+L = positive linear response at P ≤ 0.05; NS = non-significant.
Increasing EC caused shorter marigolds in ![]() experiments (Figure 1(A)); while shoot weight was not affected. EC modifications using various
experiments (Figure 1(A)); while shoot weight was not affected. EC modifications using various ![]() ratios did not affect tomato or sunflower height or shoot weight (data not shown).
ratios did not affect tomato or sunflower height or shoot weight (data not shown).
3.2. ![]() /P
/P
Marigold height decreased as ![]() /P ratio increased in the first replication (Spring, 2010; Table 3). Shoots of marigolds supplied with the highest
/P ratio increased in the first replication (Spring, 2010; Table 3). Shoots of marigolds supplied with the highest ![]() /P ratio of 60 weighed significantly less than those supplied with any other
/P ratio of 60 weighed significantly less than those supplied with any other ![]() /P treatment, including the control (Table 3).
/P treatment, including the control (Table 3).
These effects were not evident in the second marigold or either tomato replication (Spring to Summer, 2010; data not shown).
Marigolds supplied with 4.0 dS∙m−1 solutions were shorter than those supplied with lower EC solutions (Figure 1(B)) using different ![]() /P ratios. Marigold shoot weight was lowest when plants were supplied with 0.6 dS∙m−1 solutions (Figure 2(A)). At lower ECs (0.6 and 1.2 dS∙m−1) shoot dry weight decreased with increasing
/P ratios. Marigold shoot weight was lowest when plants were supplied with 0.6 dS∙m−1 solutions (Figure 2(A)). At lower ECs (0.6 and 1.2 dS∙m−1) shoot dry weight decreased with increasing ![]() /P levels (Figure 3(A)). However, this effect was absent at higher ECs (2.2 or 4.0 dS∙m−1; data not shown).
/P levels (Figure 3(A)). However, this effect was absent at higher ECs (2.2 or 4.0 dS∙m−1; data not shown).
Like marigolds, sunflowers were generally shortest at an EC of 4.0 dS∙m−1 (Figure 1(C)) in the ![]() /P experiments. Sunflower height also decreased linearly with increasing
/P experiments. Sunflower height also decreased linearly with increasing ![]() /P ratios at an EC of 0.6 dS・m−1 (Figure 3(B)), but not at the higher ECs (data not shown). Sunflower shoot weight decreased linearly with increasing NO3−/P ratios at ECs of 0.6 and 1.2 dS∙m−1 (Figure 3(A)) but not at the higher ECs (data not shown).
/P ratios at an EC of 0.6 dS・m−1 (Figure 3(B)), but not at the higher ECs (data not shown). Sunflower shoot weight decreased linearly with increasing NO3−/P ratios at ECs of 0.6 and 1.2 dS∙m−1 (Figure 3(A)) but not at the higher ECs (data not shown).
Tomatoes responded in an opposite direction to EC levels compared to marigolds and sunflowers, as tomatoes tended to be taller at higher ECs with differing ![]() /P ratios (Figure 1(D)). Tomato shoots weighed the least at an EC of 0.6 dS∙m−1 compared with the higher EC levels (Figure 2(B)). Shoot weight decreased linearly as ratio increased, but only at the lowest EC of 0.6 dS∙m−1 (Figure 3(C)).
/P ratios (Figure 1(D)). Tomato shoots weighed the least at an EC of 0.6 dS∙m−1 compared with the higher EC levels (Figure 2(B)). Shoot weight decreased linearly as ratio increased, but only at the lowest EC of 0.6 dS∙m−1 (Figure 3(C)).
3.3. ![]() /P
/P
Modifications in NH4+/P ratios had no significant effect on plant height at an EC of 1.2 dS∙m−1 (data not shown). However, marigolds were generally shorter at higher ECs using these ratios (Figure 1(E)). Dry shoot weight of marigolds at ECs of 0.6 and 4.0 dS∙m−1 weighed less than shoots from 1.2 or 2.2 dS・m−1 (Figure 2(C)). A similar trend was found for tomato shoot dry weight (Figure 2(D)) and sunflower shoot dry weight (Figure 2(E)). Sunflower height was not affected by EC level, but by ![]() /P ratio alone (Table 4). The tallest plants were obtained at a
/P ratio alone (Table 4). The tallest plants were obtained at a ![]() /P ratio of 3.0, and the shortest at a
/P ratio of 3.0, and the shortest at a ![]() /P ratio of 4.0 (1.00 mM
/P ratio of 4.0 (1.00 mM ![]() /0.25 mM
/0.25 mM![]() ).
).
![]()
Figure 1. The effect of electrical conductivity (EC) of nutrient solutions on mean height ± se using different ![]() ratios (mM/mM) for marigold (A―Winter and Spring, 2011); or using different
ratios (mM/mM) for marigold (A―Winter and Spring, 2011); or using different ![]() /P ratios for marigold (B―Summer 2011), sunflower (C―Summer 2011) and tomato (D―Summer 2011); or using different
/P ratios for marigold (B―Summer 2011), sunflower (C―Summer 2011) and tomato (D―Summer 2011); or using different ![]() /P ratios for marigold (E―Summer 2012). Data represent means from 5 ratios, 2 blocks and 2 seasons; n = 20. The following ratios were used (mM/mM): 10, 15, 20, 30 and 60 for
/P ratios for marigold (E―Summer 2012). Data represent means from 5 ratios, 2 blocks and 2 seasons; n = 20. The following ratios were used (mM/mM): 10, 15, 20, 30 and 60 for ![]() /P; 1, 1.5, 2.0, 3.0 and 4.0 for
/P; 1, 1.5, 2.0, 3.0 and 4.0 for ![]() /P and 3.25, 4.7, 7.5, 16.0 and ∞ for
/P and 3.25, 4.7, 7.5, 16.0 and ∞ for![]() . Different letter(s) above error bars within one species/ratio grouping denote significant difference at P ≤ 0.05.
. Different letter(s) above error bars within one species/ratio grouping denote significant difference at P ≤ 0.05.
![]()
Table 3. Marigold height (cm) and shoot dry weight (g) at flowering (Spring, 2010) in nutrient solutions with varying ![]() /P ratios (mM/mM) at an EC of ~1.2 dS∙m−1.
/P ratios (mM/mM) at an EC of ~1.2 dS∙m−1.
aMeans followed by the same letter within a column are not significantly different at P ≤ 0.05. bControl. cStandard error (se) with n = 2. d−L = negative linear response at P ≤ 0.05; NS = non-significant.
![]()
Table 4. Sunflower height (cm) at flowering (Spring and Summer, 2012) in nutrient solutions with varying ![]() /P ratios (mM/mM) at ECs of 0.6, 1.2, 2.2 and 4.0 dS∙cm−1.
/P ratios (mM/mM) at ECs of 0.6, 1.2, 2.2 and 4.0 dS∙cm−1.
aThe results of the different EC’s were combined as there was no statistical differences between the EC levels. bMeans followed by the same letter(s) within a column are not significantly different at P ≤ 0.05. cControl. dStandard error (se) with n = 16.
![]()
Figure 3. The effect of ![]() /P ratio (mM/mM) of nutrient solutions on shoot dry weight (g) using EC’s of 0.6 and 1.2 dS∙m−1 for marigold (A―R2 = 0.62) and sunflower (A―R2 = 0.24); or height (g) using an EC of 0.6 dS∙m−1 for sunflower (B―R2 = 0.30); or shoot dry weight using an EC of 0.6 dS∙m−1 for tomato (C―R2 = 0.33). Data represent means from 2 blocks and 2 seasons; n = 4.
/P ratio (mM/mM) of nutrient solutions on shoot dry weight (g) using EC’s of 0.6 and 1.2 dS∙m−1 for marigold (A―R2 = 0.62) and sunflower (A―R2 = 0.24); or height (g) using an EC of 0.6 dS∙m−1 for sunflower (B―R2 = 0.30); or shoot dry weight using an EC of 0.6 dS∙m−1 for tomato (C―R2 = 0.33). Data represent means from 2 blocks and 2 seasons; n = 4.
3.4. Solution EC/pH
The results of the EC and pH measurements at the beginning of each week showed that the pH decreased with increased EC (Table 5). The pH at EC = 4.0 dS/m was about 1.1 pH unit lower for ![]() and
and ![]() /P ratios and about 0.4 pH unit for
/P ratios and about 0.4 pH unit for ![]() /P ratios compared to solutions at EC = 0.6 dS/m. The results at the end of each week showed that at low EC (0.6 and 1.2 dS/m), the EC decreased, but increased when the EC was 2.2 and 4.0 dS/m compared to the initial EC for all crops. The weekly change in pH showed an increase at initial low, while it increased at the higher EC levels (2.2 and 4.0 mS/cm) for all three ratios of
/P ratios compared to solutions at EC = 0.6 dS/m. The results at the end of each week showed that at low EC (0.6 and 1.2 dS/m), the EC decreased, but increased when the EC was 2.2 and 4.0 dS/m compared to the initial EC for all crops. The weekly change in pH showed an increase at initial low, while it increased at the higher EC levels (2.2 and 4.0 mS/cm) for all three ratios of![]() ,
, ![]() /P and
/P and ![]() /P and/or species.
/P and/or species.
4. Discussion
Growers generally believe that plant height can be controlled by increasing ![]() ratio. In this study,
ratio. In this study, ![]() ratio did not reliably affect plant height or shoot weight. Nelson [10] hypothesized that the industrial misconception that
ratio did not reliably affect plant height or shoot weight. Nelson [10] hypothesized that the industrial misconception that ![]() ratio affects plant height may be attributable to the low P concentrations present in high
ratio affects plant height may be attributable to the low P concentrations present in high ![]() fertilizers.
fertilizers.
Studies suggest that low P concentrations [7] or high N/P ratios [8] may limit plant height. The ![]() /P ratios used in this study did not reliably limit plant height, but reduced shoot weight in all species, especially at a combination of low EC and high
/P ratios used in this study did not reliably limit plant height, but reduced shoot weight in all species, especially at a combination of low EC and high ![]() /P ratio (Figure 3), suggesting that growth restricting responses were the re-
/P ratio (Figure 3), suggesting that growth restricting responses were the re-
![]()
Table 5. Mean change in EC (dS∙m−1) and pH over one week periods (6) for different species during the growth from transplanting to flowering. Solution was flushed and refilled with fresh solutions on a weekly basis. N = 12 (6 weeks and 2 replications).
aInitial EC values at the beginning of each week; bInitial pH values at the beginning of each week; cChange from initial EC value over the course of one week; (+) denotes increase in value, (−) denotes decrease in value; dChange from initial pH value over the course of one week; (+) denotes increase in value, (−) denotes decrease in value; eThe ratios (mM/mM) for ![]() were 3.25, 4.7, 7.5, 16.0 and ∞; for
were 3.25, 4.7, 7.5, 16.0 and ∞; for ![]() /P were 10, 15, 20, 30 and 60; and for
/P were 10, 15, 20, 30 and 60; and for ![]() /P 1.0, 1.5,2.0, 3.0 and 4.0.
/P 1.0, 1.5,2.0, 3.0 and 4.0.
sult of absolute P concentration, rather than N/P ratio. At ECs of 0.6 and 1.2 dS∙m−1, a ![]() /P ratio of 60 provided 0.06 and 0.13 Mm
/P ratio of 60 provided 0.06 and 0.13 Mm ![]() respectively; higher concentrations than that used by Kavanova [7] to limit grass leaf elongation (0.02 mM
respectively; higher concentrations than that used by Kavanova [7] to limit grass leaf elongation (0.02 mM![]() ).
). ![]() /P ratio effects were absent (tomatoes and marigolds; data not shown) or small for sunflowers (Table 4), likely because the lowest
/P ratio effects were absent (tomatoes and marigolds; data not shown) or small for sunflowers (Table 4), likely because the lowest ![]() concentration for this set of ratios was 0.13 mM
concentration for this set of ratios was 0.13 mM![]() .
.
The effects of all three ratios (![]() ,
,![]() /P and
/P and ![]() /P) varied mostly during Spring. No ratio effects were observed during Fall or Winter months, when plant growth was generally reduced. This observation would require more study.
/P) varied mostly during Spring. No ratio effects were observed during Fall or Winter months, when plant growth was generally reduced. This observation would require more study.
High EC levels consistently produced the shortest marigold and sunflower plants for all three ratios. The exception was tomato, which increased in height with increasing EC using different ![]() /P ratios. Alternately, EC effected shoot weight similarly for all species (Figures 2(A)-(E)). Plants grown at an EC of 0.6 dS∙m−1 generally weighed the least, followed by those at an EC of 4.0 dS∙m−1. Unlike ratio modifications, EC effects were not dependent on season.
/P ratios. Alternately, EC effected shoot weight similarly for all species (Figures 2(A)-(E)). Plants grown at an EC of 0.6 dS∙m−1 generally weighed the least, followed by those at an EC of 4.0 dS∙m−1. Unlike ratio modifications, EC effects were not dependent on season.
These studies were conducted using hydroponic solutions in order to eliminate possible effects of matrix potential or interactions with the cation exchange capacity of soil and/or organic matter. However there were changes of the EC and pH due to differential uptake of nutrients and/or water by the plants over time compared to the original solutions. Effort was taken to change the solution on a weekly basis to compensate for this. However, despite the effects from differential uptake of cations, anions and concentration of ions, macronutrient modification, at the ratios evaluated in this study, would not be a viable means for plant height control in a hydroponic nutrient solution.
5. Conclusion
Contrary to industrial beliefs, plant height was not reliably affected by ![]() ratio modification. P limitation (high
ratio modification. P limitation (high ![]() /P ratios) more consistently resulted in decreased shoot weight rather than plant height. P concentrations lower than those used in this hydroponic study (<0.06 mM
/P ratios) more consistently resulted in decreased shoot weight rather than plant height. P concentrations lower than those used in this hydroponic study (<0.06 mM![]() ) may be required to achieve more consistent height control. Our results show that plant height control, depending on species, may be better attained through increasing EC levels rather than nutrient modification alone.
) may be required to achieve more consistent height control. Our results show that plant height control, depending on species, may be better attained through increasing EC levels rather than nutrient modification alone.
Acknowledgements
This research project was financially supported by Flowers Canada (Ontario) and the Canadian Ornamental Horticulture Research and Innovation Cluster.