Production of Synthesis Gases from Ethanol Steam Reforming Process ()
Received 12 July 2016; accepted 14 August 2016; published 17 August 2016

1. Introduction
Due to high demand in energy consumption and the recent improvements in fuel cell technology, it increases the synthesis gas production requirements from the different fuel sources. Hydrogen from steam reforming of ethanol was studied by F. J. Marino et al. [1] . The effect of copper loading and calcination temperature on the structure of the catalyst were studied. In order to maximize hydrogen production, copper loading, calcination temperature and catalyst preparation were purposed [1] . A thermodynamic analysis of hydrogen production by steam reforming of ethanol was studied by I. Fishtik et al. [2] . A simple algorithm was purposed to rationalize the effect of process variables on steam reforming of ethanol. At or above 700˚K - 800˚K and higher water/ ethanol ratios, the desired reaction of ethanol steam reforming reaction can be predominated [2] .
A two-layer fixed bed catalytic reactor for syngas production by steam reforming of ethanol was reported by V. V. Galvita et al. [3] . In recommended concept, ethanol is, firstly, converted to a mixture of methane, carbon oxides and hydrogen and then this mixture is converted to syngas [3] . Hydrogen production by catalytic ethanol steam reforming was studied by A. Therdthianwong et al. [4] . In this study, experiments were conducted in a fixed bed reactor on Ni/Al2O3 catalyst. As W/F ratio and temperatures increase, the H2 yields increase in reactor exit. For rate expression, the power-law rate expression was determined.
Hydrogen generation by steam reforming of methanol over copper-based catalysts for fuel cell applications was studied by B. Lindström et al. [5] . In the research, it was concluded that copper-based catalysts were effec- tive for the steam reforming of methanol. The use of copper content and promoters (Cr, Zn, Zr) played an important role in converting selctively methanol at low temperatures (180˚C - 320˚C). Optimisation of ethanol reforming for hydrogen production in a hybrid electric vehicle was purposed by V. Klouz et al. [6] . A detailed kinetic scheme of the ethanol was discussed as a function of the temperature and the role of oxygen in the reaction selectivity and coke formation was investigated.
H2 production for molten carbonate fuel cell from steam reforming of ethanol was studied by S. Freni et al. [7] . Results of the study indicated that Ni-MgO catalytic systems were adequate properties for efficient hydrogen production by ethanol steam reforming. Conversion of hydrocarbons and alcohols for fuel cells was studied by Joensen et al. [8] . Pure hydrogen is the recommended fuel due to simplicity in design, low cost and high efficiency [8] .
The reformation of biomass-derived ethanol to a hydrogen-rich gas stream was studied by A. N. Fatsikostas [9] . The influence of reaction temperature, water to ethanol ratio and space velocity was conducted. Hydrogen production by auto-thermal reforming of ethanol on Rh/Al2O3 catalyst was studied by S. Cavallaro [10] . The results have indicated that if the optimum range where ethanol conversion is 100% is reached, the productions of CH4 and CO go down and hydrogen yield reaches to the maximum range before decreasing.
The catalytic performance of supported noble metal catalysts for the steam reforming of ethanol has been investigated by D. K. Liguras et al. [11] in the temperature range between 600˚C - 850˚C. They concluded that supported Rh catalysts were significantly more active and were selective for ethanol steam reforming reaction. The metal-support interaction triggering Cu-Ni-K/γ-Al2O3 supported catalysts for ethanol steam reforming formation of hydrotalcite-type compounds was studied by F.Marino et al. [12] .
The function of the steam reforming of ethanol on 5% Rh/Al2O3 catalyst in producing H2 to feed a molten carbonate fuel cell was investigated by S. Cavallaro et al. [13] . The ethanol steam reforming on Ni/γ-Al2O3 catalyst at temperature between 573 and 773˚K was studied by J.Comas et al. [14] and an overall reaction scheme as a function of temperature was purposed. They concluded that high temperatures (above 773˚K), higher water/ethanol ratios (about 6:1) promote high hydrogen yield on Ni/γ-Al2O3 catalyst.
The effects of alkali additions (e.g. Li, Na, K) on the behavior of Ni/MgO catalyst in the bio-ethanol steam reforming have been investigated by F. Frusteri et al. [15] . The effects of catalyst synthesis method (e.g. pre- cipitation, coprecipitation and impregnation), Ni loading and reduction temperature on the characteristics and performance of Ni/Al2O3 catalysts were studied by A. J. Akande et al. [16] with the aim of investigating the reforming of crude ethanol for H2 production. As a result, it was found out that precipitation catalysts were more reducible than coprecipitation and impregnation catalysts. Catalyst with 15% Ni loading trigger the best crude ethanol conversion [16] .
By means of ultra high vacuum temperature which is programmed desorption conditions, the influence of oxygen on the decomposition of ethanol over rhodium and single crystal surfaces were studied by E. Vesselli et al. [17] . On the contrary, excess of oxygen lead to a strong reduction of hydrogen concentration in the reaction products. Influence of the addition of promoters to steam reforming catalysts was studied by J. S. Lisboa et al. [18] . The catalyst was Ni/α-Al2O3 which was modified by the addition of promoters (Mgand Ca) to improve their stability and selectivity. The results of reaction demonstrated that there is an increase in the reforming activity by the presence of promoters. A series of CuNiZnAl-multicomponent mixed metal oxide catalysts was used in the oxidative steam reforming of bio-ethanol by S. Velu et al. [19] . As a result, Cu2+ was fully reduced, while Ni2+ and Zn2+ were partially reduced.
Ethanol steam reforming over MgxNi1−xAl2O3 spinel oxide-supportedRh catalysts was studied by F. Aupretre et al. [20] . In this study, the effects of the precursors which were used in the support and catalyst preparation were conducted. Nitrate precursors were the most obtained acidic materials and had the poorest stability one. The catalyst prepared with Rh chloride were moderately acidic, and they were very active and stable. Ethanol steam reforming consists of three main reactions accounting for the formation of H2, CO2, CO, and CH4 [20] :
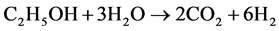 , (1)
, (1)
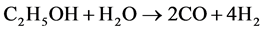 , (2)
, (2)
 . (3)
. (3)
Three main parameters may affect the H2 yield at equilibrium (YH2): temperature (T), total pressure (P), initial H2O/ethanol molar ratio (FWE). YH2 increases with T and FWE and decreases with pressure [20] .
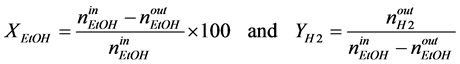 . (4)
. (4)
The influence of the nature of the metal on the performance of cerium oxide supported catalyst in the partial oxidation of ethanol was studied by L. V. Mattos and co-worker [21] . The results which were obtained from the study demonstrated that nature of the metal strongly affected the product distribution.
M. Benito and co-workers [22] have studied a new catalyst for hydrogen production by steam reforming of bio-ethanol. Three catalysts have been synthesized and tested for ethanol steam reforming. One of the developed catalysts was very active with 100% ethanol conversion at 700˚C. Ethanol reforming over Ni/MgO and Ni/CeO2 catalyst in molten carbonate fuel cell has been investigated by F. Frusteri et al. [23] . Both catalytic systems, at equilibrium conditions, are able to produce a rich hydrogen stream.
A global perspective of recent advances on the production and utilization trends of bio-fuels was referred by M. F. Demirbaş [24] . Hydrogen production by ethanol reforming over NiZnAl catalysts was studied by M. N. Barroso et al. [25] . They have obtained a complete ethanol conversion at 500 and 600˚C. The product dis- tribution depends on nickel loading the catalysts with Ni amounts between 18 and 25 wt.% which they are the ones showing the best performance [25] .
Ceria-supported Co, Ir and Ni catalysts were investigated for steam reforming of ethanol in the temperature of 300˚C - 700˚C by B. Zhang et al. [26] . It was demonstrated that ceria supported catalyst were significantly active and selective for hydrogen production by steam reforming of ethanol. The strong interaction between Ir and CeO2 effectively prevented the sintering and remarkably facilitated coke gasification [26] . L. Dolgykh et al. [27] studied the catalytic performance of Cu-containing industrial dehydrogenation catalyst for the steam reforming of ethanol using 12 wt% ethanol in water mixtures. The catalyst which was used in the study made achievement of hydrogen at 250˚C - 300˚C possible.
A. Akande et al. [28] studied kinetic modeling of hydrogen production by the catalytic reforming of crude ethanol over a Ni-Al2O3 catalyst in a packed bed tubular reactor. A few kinetic models was purposed. A power law model was also tried to fit the experimental data. Hydrogen production from ethanol reforming over alumina-supported nickel catalysts modified with Ce, Mg, Zr and La was studied by M. C. S. Sanchez et al. [29] . The activity of the catalysts was explained in terms of the different acidity, nickel dispersion or support-nickel interaction.
The effect of the morphology of nanocrystalline CeO2 on ethanol reforming was studied by W. I. Hsiao et al. [30] . M. Ni et al. [31] carried out a research on reforming bio-ethanol for hydrogen production. They concluded that Ni and Rh based catalyst is the best choice for bio-ethanol conversion and hydrogen selectivity.
A review of bio-ethanol to produce hydrogen for a molten carbonate fuel cell was written by F. Frusteri and his co-worker [32] . This review demonstrated that the presence of low amount of oxygen in the reaction stream pozitively affects both activity and stability of catalyst.
A short communication about novel zeolite-supported rhodium catalyst for ethanol steam reforming was given by F. C. C. Skrobot et al. [33] . In conclusion, NaY zeolite-supported Rh has been suggested for ethanol- steam reforming. P. Biswas and D. Kunzru [34] studied catalytic steam reforming of ethanol both in presence and absence of oxygen over Ni-CeO2-ZrO2 catalyst. As a result, hydrogen yields were significantly affected by oxygen. In the absence of oxygen, higher hydrogen yield were obtained at higher temperatures.
Steam reforming of ethanol over Ni/support catalyst for generation of hydrogen was studied by A. Denis et al. [35] . It was found out that the most suitable supports in nickel catalysts designed for hydrogen generation in the steam reforming of ethanol are ZnO and TiO2. Steam reforming of ethanol over an Ir/CeO2 catalyst has been studied by B. Zhang et al. [36] . They have investigated the reaction mechanism and stability of the catalyst. They have concluded that the Ir/CeO2 catalyst is significantly active for ethanol steam reforming and demon- strates good stability.Effect of Mg addition on ethanol steam reforming over Ni/Al2O3 catalysts was investigated by A. J. Vizcaino et al. [37] . The results demonstrated that Mg addition to Ni precursor leads to higher activities of prepared catalysts with an important reduction in the amount of deposited coke.
Hydrogen production through methanol steam reforming over Ni-Cu/CaO-SiO2 catalysts activity was studied by Y. Ren-Xuan et al. [38] A modified polyol process conducted under an Ar atmosphere at 160˚C can successfully synthesize Ni-Cu/CaO-SiO2 catalysts, which are effective in SRM to produce H2. Hydrogen production from n-butanol over alümina and modified alumina nickel catalysts was studied by K. Bizkarra et al. [39] . In that sudy modified supports and catalysts were prepared by wet impregnation method, and tested and compared with a commercial catalyst. They found some deactivation signs and between all tested catalysts the Ni/CeO2-Al2O3 provided the highest hydrogen.
Thermodynamic and an experimental study of production of hydrogen from the steam and oxidative reforming of LPG was carried out by P. P. Silva et al. [40] . Their results showed that it is possible to produce high concentrations of hydrogen from LPG reforming. The gradual increase of temperature and the use of high water concentrations decrease the production of coke and increase the formation of H2. Rh/Al2O3-La2O3 catalysts promoted with CeO2 for ethanol steam reforming reaction was studied by P. Osorio-Vargasa et al. [41] . Rh-based catalysts supported on Al2O3-La2O3 modified with CeO2 promoter were prepared. They reporting that catalyst exhibited good selectivity to H2, with no presence of C2 intermediates above 723˚K. Reactivation tests showed that it is possible to recover part of the activity of the original catalyst after subjecting it to a pre- treatment by oxidation and reduction in order to remove any carbon deposits.
Syngas production from CO2 reforming of methane over ceria supported cobalt catalyst and effects of reactants partial pressure was studied by B. V. Ayodele et al. [42] . The findings from this study show that syngas production from methane dry reforming over ceria-supported cobalt catalyst is significantly influenced by the variation in the partial pressure of the reactants.Their catalytic activity test shows that highest CO2 and CH4 conversions were obtained at CH4 partial pressure of 45 and 25 kPa. Production of hydrogen from steam reforming of ethanol over LaNiO3 and LaNiO3/CeSiO2 oxide types catalysts was studied by A. L. A. Marinhoa et al. [43] . Their results showed that LaNiO3/CeSiO2 is a promising catalyst for H2 production from ethanol, since their material exhibited high activity and lower carbon formation during SR of ethanol at 773˚K. Hydrogen production by catalytic coal gasification and hydrogen production by ethanol steam reforming in two fixed bed reactors over eutectic salts(Li2CO3, Na2CO3 and K2CO3) and over a commercial BASF catalyst were studied by M. Levent et al. [44] - [48] . As a result, higher hydrogen ratios have been obtained at 5% - 10% eutectic salts and at lower ethanol/water ratios.
In this study, experimental studies were conducted under different temperatures, catalyst quantities and different state spaces over a commercial BASF catalyst and a laboratory prepared catalyst. In the case of commerical BASF catalyst hydrogen production rates were higher for similar experimental conditions of laboratory prepared catalyst. But, at temperatures over 700˚C using commercial BASF catalyst for production of H2 was not con- venient, because H2 production rates were reduced and some coke depositions were detected on the catalyst surfaces and deformation of catalyst was detected. In the case of using laboratory prepared catalyst up to 700˚C, H2 production rates were slightly lower (5% - 10%) than commercial catalyst. However, at temperatures over 700˚C, better stabilities of laboratory catalyst have detected, and laboratory prepared catalyst were used for long periods and less carbon quantities are detected on the catalyst sufaces and H2 prouduction rates were slightly reduced over 700˚C. At lower temperatures less than 700˚C, using commercial catalyst has some advantages, but at temperatures higher than 700˚C, using laboratory catalyst have some advantages about activity and stabi- lity. By carriying out some experiments at higher temperatures higher than 700˚, some important achivements and parameters were determined for industrial operation conditions of H2 productions. At higher temperatures more than 700˚C, some increments in production rate of CO were detected and a small decrement in H2 rate and some decrements in CO2 rate have been observed.
2. Experimental System
Experimental system was conducted in four sections (parts) (Figure 1): 1) Flow System: It contains 4 rotameters and each one has a 0 - 1000 ml/min of measurement capacity.
2) Steam system: It can be run in 0 - 4 bars operational pressure conditions; it has 0 - 6 ml/min water feeding capacity of one perilstatic pump which was made of nickel-chromia alloy steel. The steam system has a 1.5 m vertical pipe in length and it consists of connectors to reactor and pump, steam safety valve and one pressure gauge. Perilstatic pump is located in the bottom of the system. Water is pumped into the oven in reverse direction and it flows by evaporating into the reactor from top of the oven.
3) Reactor System: Two fixed bed reactors with higher temperature prof of chromia-nickel alloy, each one has 2 cm external, 1.5 cm internal diameters and each reactor has 93 cm and 83.5 cm in length, respectively [44] - [48] . Tubular oven has a cubic shape in external part and it is completely insulated against any temperature losses. The oven has a 5 cm internal diameter and 80 cm length. The ceramic pipe is completely rolled by a resistance wire material. This oven is covered with fire cement refracter. It can be heated up with 380 volts industrial electrical voltage and can be heated up to 1200˚C temperature.
4) Gas analysis system is made up of two parts (units). A). Gas mixing system which is able to make up various gas mixtures. This system consists of 4 rotameters, each rotameter has a capacity of 0 - 1000 ml/min. Each of these rotameters is calibrated separately with pure H2, CH4, CO and CO2 gases [38] - [42] . B). Midi GC (Model 1001 from Pherichrom company, Paris, France) gas analysis equipment has a 15 kg weight and a cubic shape equipment. There was a local control panel in front of the equipment. There was a TCD detector in the
![]()
Figure 1. Flow diagram of experimental system (This experimental setup was designed by M. Levent for two completed projects (TÜBİTAK-MAG No: 106M162 and AÜ BAP No: 2004/101) [44] - [48] ).
equipment. There were two packed columns in the equipment where one of them was porapack Q and the other one was molecular sieve column. The configuration of equipment was adjusted to do the analysis of exit gases in hydrocarbon reforming process. There are two automatic valves next to the equipment. One of the valve was commutation and the other one was the equilibrium valve [44] - [48] .
Before starting to experimental studies, 1 - 5 grammes catalysts had been loaded into the reactor. In order to prevent any carbon deposition on catalyst surface, the catalyst had been conditioned at 500˚C with 100 ml/min H2 flow for 6 hours. Thus, NiO in the catalyst was reduced to metalic nickel. The activity of catalyst was pro- vided [44] - [48] .
At the beginning of the experiment, 200 ml/min N2 gas was fed to the system. When system temperature reached to 250˚C, nitrogen and hydrogen flows had been cutted off. In order to prevent catalyst surface from any coke formation, the conditioning experiments were carried out for designated intervals every day. The catalyst deformation related to the transmitted water droplets to the catalyst bed was prevented via heating the EtOH/ water mixtures up to higher temperatures. After the reduction of the catalyst with H2, in order to prevent the catalyst from any carbon deposition, the experiments were conducted continously [44] - [48] .
After the preparation of the catalyst for experiments, 0.5 - 1 ml/min EtOH/water mixtures were fed to the system. Temperature was raised gradually and in different temperatures the gas compositions were analyzed at the exit of the system. The system has a facility providing to analyse all the gases in the present study. Helium and nitrogen were used as carriers for GC. The exit voltages of GC analysis equipment were read continously through an ADC card. The exit voltages were continously observed in a monitor of Pentium-IV computer by the aid of a drawing program which was developed recently by Dizge analytical Co., Ankara, Turkey. All output peaks related to each experiment were stored in the computer. Then, peak areas of each gas in unknown samples were determined by GC and they were recorded in computer. By comparison of unknown samples peaks with pure gas samples, the composition of each component in unknown samples were determined [44] - [48] .
The inlet pressure of carrier gas to GC was 2.1 bars and the temperature of GC oven was 80˚C. While doing analysis with helium, detector current was 150 mA and accuracy was adjusted to 10. While analysing gas mixtures with nitrogen, detector current was adjusted to 60 mA and accuracy (gain) was adjusted as 10. GC accuracy balances were changed between 1 to 10. The value of 10 was the most accurate value of balance and 1 was the least accuracy value of gain (M. Levent, et al. [44] - [48] ).
In the third stage of experimental programme, the oven resistance was replaced with a new resistance having a capacity of operating up to 700˚C. Then, the rest of the experimental measurements were conducted at tem- peratures up to 700˚C. Higher temperature operational conditions haven’t been tried any more. All the remain experiments were carried out at temperatures between 250˚C - 700˚C and at catalyst loads of 1 to 5 grammes and at different ethanol/water ratios. As a result, through using lower ethanol/water ratios, higher hydrogen percentages were determined at the exit of the reactor system (see Figures 2-9).
2.1. The Composition of the Commercial BASF Catalyst and Catalyst Test Studies
In this study, the used commercial BASF catalyst was contained higher ratios of NiO. The catalyst has a 52% NiO, and other supports or promoters materials were SiO2, MgO, CaO and Cr2O3. The catalyst has a cylindrical tablet shape with 3 mm height and 3 mm diameter. The catalyst density was 1006 kg/m3 and it can be used between 0 to 700˚C operation temperatures. The specific surface area of catalyst was 247 m2/gr. Surface area measurement and trace element analysis of the catalyst were carried out with a BET equipment (Model: ASAP 2000), XRD and WDXRF equipments both at the beginning and at the final step of this study by taking per- mission from BASF company. In the initial step of tests, coke (carbon) couldn’t be seen in the catalyst content. After one month study period, some carbon deposites were detected on the surface of the used catalyst. This is an indication demonstrating that a trace quantity of coke was formed on the catalyst surface during each experi- ment. But, the coke formation was very slow and the catalyst could only be used for one month. As a result, significant decline in hydrogen yields couldn’t be observed in one month study period. In order to prevent the catalyst surface from any coke deposition, the reaction environment was kept in reductive conditions by providing a small stream of hydrogen through the reactor content.
2.2. The Composition of the Laboratory Prepared Catalyst and Catalyst Test Studies
In this study, a laboratory prepared catalyst which contains basically NiO, Al2O3 support and promoters such as
![]()
Figure 2. Reactor exit H2 percentages for various EtOH/H2O ratios against temperature for commercial BASF catalyst [45] [47] [48] .
![]()
Figure 3. Reactor exit H2 percentages for various EtOH/H2O ratios against temperature for commercial BASF catalyst [45] [47] [48] .
![]()
Figure 4. Reactor exit H2 percentages for 1/8 EtOH/H2O value against commercial BASF catalyst quantity [45] [47] [48] .
![]()
Figure 5. Exit H2 percentages for various EtOH/H2O ratios against temperature for commercial BASF catalyst [45] [47] [48] .
![]()
Figure 6. Exit H2 percentages for various EtOH/H2O ratios against temperature for commercial BASF catalyst.
![]()
Figure 7. Exit H2 percentages for 1/8 EtOH/H2O value against laboratory prepared catalyst quantity [45] [47] [48] .
![]()
Figure 8. Exit H2 percentages for various EtOH/H2O ratios against temperature for laboratory prepared catalyst.
![]()
Figure 9. Exit H2 percentages for various EtOH/H2O ratios against temperature for laboratory prepared catalyst. [45] [47] [48] .
SiO2, MgO, CaO and Cr2O3 was synthesized. For this purpose, a wighed quantity of nickel nitrate (NiNO3) salt was dissolved in distilled water. Then, it was added into a certain quantity of Al2O3 drop by drop. Then, it was stirred with a digital magnetic stirrer (Heidolph Mark, Model MR 3003 SD) and a digital mechanical stirrer (IKA Werk Mark, Model RE-166) at the speed of 300 rev./min. Simultaneously, other promoters (SiO2, MgO, CaO and Cr2O3) were added into the same solution and it was stirred for an hour. In order to remove NO3 deposits in calcination step without encountering any trouble, the pH of mixture was kept around 5,5 through adding an aqueous solution of NaOH drop by drop. The mixture of solution was filtered and dried in an oven. Then, it was calcined at 400˚C. Then, the obtained catalyst powder was pelleted with a laboratory scale hydrolic press. The BET surface area of laboratory prepared catalyst was 115 m2/gr. SEM analysis of the laboratory prepared catalyst was also carried out. Then, catalyst pellets was used in the reactor. Some of the reaction runs were conducted with crushed catalyst particles (0.30 - 0.40 µm) of commercial BASF and laboratory prepared catalyst.
This laboratory prepared catalyst has a slightly less activity than commercial BASF catalyst. All reaction runs were carried on between 300˚C to 700˚C temperatures. As a result, the laboratory prepared catalyst has a longer life time than commercial BASF catalyst. Activity of commercial BASF catalyst was found slightly better than laboratory prepared catalyst.
3. Results and Discussions
In this study, hydrogen production yields were investigated at different EtOH/water ratios and at different temperatures with ethanol-water vapour process over a commercial BASF and a laboratory prepared catalyst. In this experimental study based on volume ratios of 1/10, 1/8, 1/6, 1/4 and 1/2, ethyl alcohol/water ratios at different temperatures (300, 400, 500, 600, 700˚C) obtained peak areas of hydrogen and produced H2 % values have been computed.
A new catalyst (NiO/Al2O3) promoted by CaO, MgO and Cr2O3 was prepared and it was usedfor H2 pro- duction under similar experimental conditions of commercial BASF catalyst. The laboratory prepared catalyst has demonstrated higher activity values (upto 70% H2 values). And longer catalyst life time was observed during ethanol-steam reforming reaction. The hydrogen yields in the exit gas composition increased slightly in paralel with the increase of catalyst loads. The hydrogen yields in exit gas composition also increased with the increase in temperature and decrease in EtOH/water ratios. The duration of laboratory prepared catalyst is longer (2 - 3 weeks) than the duration of commercial BASF catalyst (1 - 2 weeks). The maximum operation temperature of commercial BASF catalyst was 700˚C. However, the laboratory prepared catalyst has been used at higher tem- peratures (800˚C - 900˚C). In the first stage of the study, some experiments were carried out at higher tem- peratures above 700˚C. The laboratory prepared catalyst has demonstrated good activity levels even at higher temperatures over 700˚C. But, the owen resistance was burned out at higher temperature operations.
Reaction started arround 300˚C and the reaction rate was increasing proportionally with the temperature. Hydrogen yields were slightly increasing in paralel with the increases in catalyst loads. At very high tem- peratures over 700˚C up to 900˚C, the sintering and coke deposition in commercial BASF catalyst was slightly higher than laboratory prepared catalyst. The activity of laboratory prepared catalyst was longer than com- mercial BASF catalyst. But the hydrogen yields and activity of commercial BASF catalyst was slightly higher than laboratory prepared catalyst at operational temperatures up to 700˚C. The coke deposition rates in both catalyst surfaces were increasing with higher ethanol/water ratios of feed. Coke deposition on catalyst surface during 1/2 volume ratio of ethanol/water feed was higher than coke deposition during 1/10 volume ratio of EtOH/H2O feed.
H2 pecentages against temperature for 1/10 EtOH/water ratio was given in Figure 2. As it can be seen on Figure 2, H2 percentages have demonstrated a rapid increaseto over 80% at 300˚C. This indicated that EtOH/H2O reaction over BASF commercial catalyst was taken place rapidly at 300˚C to higher temperatures. Exit H2 percentages against temperatures at different EtOH/H2O ratios over 1 gr. of commercial BASF catalyst were given on Figure 2. As it can be seen on graph, the percentages of H2 were varied between 7% to 15% at 300˚C and at different EtOH/H2O ratios between 1/2 - 1/8 when temperature values increased from 400 to 500˚C, and H2 percentages were increased, sharply from a range of 7% - 15% to a range of 50% - 70%. If EtOH/H2O ratios were declined from 1/2 to 1/8 at 700˚C, then, H2 percentages were changed to a range of 60% - 70% at the exit of the reactor. As a result, H2 percentages are linearly proportional with increments in temperatures and decrements in EtOH/H2O ratios between 1/2 - 1/8 and H2 percentages were inversely proportional with decrements in temperature from 700˚C to 300˚C.
Exit H2 percentages which are ranged from 4% to 60% over 1 gram of laboratory prepared catalyst were given on Figure 3. As it can be seen on this figure, H2 percentages increased slightly from 5% to 17% at lower temperatures from 300˚C to 400˚C. When temperature was increased from 400˚C to 500˚C, H2 percentages increased sharply from the range of 5% - 17% to the range of 40% - 53%. If temperature was increased further from 500˚C to 700˚C, H2 percentages also slightly increased to the range of 50% - 60%. As a result, H2 percentages increased in the paralel with temperatures, and EtOH/H2O ratios are effective on H2 yields.
Exit H2 percentages for 1/8 EtOH/H2O ratios against quantities of commercial BASF catalyst were given in Figure 4. As seen on this graph, when the quantities of commercial BASF catalyst were increased from 0 to 5 grammes, hydrogen percentages in exit gas composition also increased linearly. H2 percentages were found around 66% - 70% in the temperature ranged from 500 to 700˚C. However, in the temperature range of 300˚C - 400˚C with lower catalyst quantities from 1 to 3 grammes, H2 percentages increased slightly from 25% to 35%. When catalyst loads were increased from 3 to 4 grammes, H2 percentages increased sharply to the range of 70% - 72%. When catalyst quantities were increased from 4 to 5 grammes, H2 percentages increased slightly to the range of 70% - 73%. As a result, H2 percentages depend on temperature increments and H2 percentages are increasing with quantities of commercial BASF catalyst.
Exit H2 percentages against different teperatures for commercial BASF catalyst were given in Figure 5. As seen on this graph, H2 percentages increase linearly with temperature. While produced H2 percentages are higher at lower EtOH/H2O ratios, produced H2 percentages are lower at higher ethanol/water ratios. H2 percentages changed in the range of 63% - 73% at 300˚C. When temperature was increased to 700˚C, the range of H2 percentage became 72% - 85%. In the lights of these findings it can be said that H2 percentages increase in the exit of the reactor paralel with temperature increments in reactor system.
H2 percentages against temperatures over 1 gram of commercial BASF catalyst were given in Figure 6. H2 percentages were increased in three steps. In first step (300 to 400˚C) , H2 percentages were increased slightly. In second step (400 to 500˚C), H2 percentages were increased sharply and in final step (500 to 700˚C), H2 percentages were increased slightly to the range of 65% - 80%.
Exit H2 percentages against laboratory prepared catalyst quantities were given in Figure 7. As seen on this graph developed for 1/8 EtOH/H2O ratio, when catalyst quantities were increased from 1 to 5 grammes, H2 percentages also increased from lower values to higher values. For the catalyst range from 1 to 3 grammes and temperature range of 300˚C - 400˚C, H2 percentages changed from the range of 29% - 32%, to the range of 33% - 35%. When catalyst loads were increased from 3 grammes to 4 grammes at same temperature range, H2 percentages increased sharply to the range of 58% - 60%. For further increments in catalyst loads at the same temperature range, H2 percentages were increased, slightly to a range of 63% - 65%. For temperature range of 500˚C to 700˚C with increments in catalyst loads from 1 to 5 grammes, linear increments in H2 percentages were achieved in the range of 60% - 65% and 65% - 75%.
Exit H2 percentages at different temperatures and different ethanol/water ratios over 5 grammes of laboratory prepared catalyst were given in Figure 8. As it can be seen on this figure, H2 percentages at 300˚C linearly increased from the range of 56% - 67% to the range of 66% - 75% at 700˚C. Exit H2 percentages at different temperatures and different EtOH/H2O ratios were given on Figure 9. In this figure H2 percentages were also increased in three steps. In first stage whose temperature ranged from 300 to 400˚C, H2 percentages increased slightly from the range of 17% - 30% to a range of 18% - 32%. In second stage whose temperature ranged between 400 to 500˚C, H2 percentages increased sharply from the range of 20% - 30% to the range of 43% - 60%. At further temperatures from 500 to 700˚C, H2 percentages increased slightly to the range of 50% - 63% at 700˚C.
Peak areas at different temperatures for 1 gram of commercial BASF catalyst and 1/8 EtOH/H2O ratio were given in Figure 10. Peak areas of H2 were linearly increased from 500 milivolts(mVs) at 300˚C to 4000 mVs at 500˚C. While peak areas of H2 were increased slightly to the value of 4300 mVs at 700˚C, The peak areas of other gases were changed between 100 to 800 mVs at temperature range of 300 to 700˚C.
Peak areas at different temperatures for 1 gram of laboratory prepared catalyst and 1/8 EtOH/H2O ratio were given on Figure 11. In this graph, it can be seen that peak areas of H2 were continously increased from 0 mVs to 3000 mVs with increasing temperature. Peak areas of H2 were increased linearly to a value of 3800 mVs,
![]()
Figure 10. Pick areas of all exit gases for 1 gramme commercial BASF catalyst and 1/8 EtOH/H2O value.
![]()
Figure 11. Pick areas of all exit gases for 1 gramme of laboratory prepared catalyst and 1/8 EtOH/H2O value [45] [47] [48] .
approximately at 700˚C. Peak areas of other gases (CO2, CH4, CO) are changed in the range of 50 - 1000 mVs, approximately.
Ethanol conversion percentages at different Space times(W/FA0) and at different temperatures (500˚C - 700˚C) for 1/8 EtOH/H2O ratio over commercial BASF catalyst were given in Figure 12. As it can be seen on this graph, ethanol conversions also increase parabolically at temperatures 500˚C and 600˚C with increment in space times. However, H2 percentages increase linearly with increment in space times at temperature of 700˚C.
Higher ethanol conversion values (in the range of 85% - 100%) at different spaces times (in the range of 0.015 - 0.060) and at 700˚C were achieved. At different spaces times (in the range of 0.015 - 0.060) and at different temperatures (500˚C and 600˚C), different ethanol conversion ranges of 86% - 88% and 92% - 93% were achieved, respectively.
Ethanol conversions at different space times (W/FA0) and at different temperatures for 1/8 EtOH/H2O ratio and laboratory prepared catalyst were given on Figure 13. As seen on this graph, ethanol conversion increase with increments in space times.
At higher temperatures (700˚C), 98% of ethanol conversion was achieved over laboratory prepared catalyst. Ethanol conversions at diffrent temperatures increase with space times (W/FA0). Peak areas (mVs) of all gases at different space times (W/FA0) for 1/8 EtOH/H2O ratio over commercial BASF catalyst were given on Figure 14. As seen on this graph, peak areas of H2 is higher than peak areas of other gases (CO2, CH4, CO). Peak areas of H2 were changed between 4000 mVs and 5000 mVs. The peak areas of other gases were changed between 50 mVs and 1000 mVs.
Peak areas of all gases at different space times(W/FA0) for 1/8 EtOH/H2O ratio over laboratory prepared catalyst were given on Figure 15. As seen on graph, peak area of H2 is higher than peak areas of other gases (CO2, CH4, CO). Peak areas of H2 were changed in the range of 3800 - 4200 mVs. However, peak areas of other gases were changed in the range of 100 mVs - 1100 mVs.
The reaction kinetic parameters can be calculated on the basis of the following equations. The derivation of the differential and integral forms of the design equations for packed bed reactors were given by H.S. Fogler [43]
![]()
![]() (5)
(5)
where ![]() is molar flowrate of ethanol, PEtOH is partial pressure of ethanol, R is gas constant, W is Catalyst weight, T is absolute temperature and X is mole fraction of each component. The activation energy is deter- mined experimentally by carrying out the reaction at several different temperatures [43] :
is molar flowrate of ethanol, PEtOH is partial pressure of ethanol, R is gas constant, W is Catalyst weight, T is absolute temperature and X is mole fraction of each component. The activation energy is deter- mined experimentally by carrying out the reaction at several different temperatures [43] :
![]() (6)
(6)
![]()
Figure 12. Ethanol conversion against space time in the reactor with different gas temperatures from obtained data at 1/8 EtOH/H2O ratio with commercial BASF catalyst [45] [47] [48] .
![]()
Figure 13. Ethanol conversion against space time in the reactor with different gas temperatures from obtained data at 1/8 EtOH/H2O ratio with laboratory prepared catalyst [45] [47] [48] .
![]()
Figure 14. Pick areas of all gases obtained at the exit of the reactor against space time in the reactor of gases from obtained data in 1/8 EtOH/H2O ratio with commercial BASF catalyst [45] [47] [48] .
![]()
Figure 15. Pick areas of all gases obtained at the exit of the reactor against space time in the reactor from obtained data of gases at the 1/8 EtOH/H2O ratio with laboratory prepared catalyst [45] [47] [48] .
where k is reaction rate constant, A is frequency factor, EA is activation energy and T is absolute temperature. In order to calculate the activation energies and determine the diffusion effects on the reaction rates, Lnk graph against inverse of absolute temperatures for 1/8 ratio of EtOH/H2O ratio over 5 grammes of commercial BASF catalyst were given in Figure 16. As seen on this graph, lnk values are inversely linear with the reverse of absolute temperatures. Diffusion effects on overall reaction rate may not be very significant. But, some indi- cations of diffusion were determined during computation of activation energies. Because, different activation energy values were obtained over different catalyst quantities. When loaded catalyst quantities were increased, the determined activation energy values were also increased significantly.
In order to calculate the activation energies and understand the diffusion effects on overall reaction rate, lnk graph against inverse of absolute temperatures for 1/8 EtOH/H2O ratio and 5 grammes of laboratory prepared catalyst was drawn on Figure 17.
As seen on graph, lnk values are inversely linear with the inverse of absolute temperatures. This means that diffusion effects related to the reaction are small and the reaction may be taken as first order. From the slope of this graph, the activation energy of ethanol-steam reforming reaction could be determined. The activation energies of different experimental runs were also calculated from Arrhenius equation [49] . Activation energy values determined from theoretical equations and activation energies determined from graphs were found in good agremeent in the range of 6.50 to 52.7 kJ/moles. Determined activation energies strongly depend on the type and quantities of the treated catalyst.
4. Conclusions
So far, some kinetic parameters related to ethanol-steam reforming reaction were determined at different temperature conditions and at different catalyst loads with different ethanol/water feed ratios. On the basis of obtained kinetic parameters, ethanol-steam reforming reaction was put in the first order and chemical reaction was controlled. The obtained activation energies have good fit with similar studies in literatüre [50] - [52] . As a result of experiments conducted at different ethanol/water ratios, at different temperatures and at different catalyst loads (1 to 5 grammes), H2 yields were computed. In the exit gas compositions, the highest hydrogen concentrations were found at lower EtOH/water feed ratios and at higher temperatures. Experimental work was completed by using different catalyst dimensions in paralel with different catalyst loads and different ethanol/ water feed ratios at different times.
Two series of experimental measurements were carried out in this study. First series of experimental measurements is performed over a commercial BASF catalyst which is perfect up to temperatures of 700˚C. The second series of experimental measurements are carried out with a laboratory prepared catalyst at temperatures up to 900˚C. Hydrogen production rates over commercial BASF catalyst are silghtly higher (5% - 10%) than hydrogen production rates over laboratory prepared catalyst up to temperatures of 700˚C. But, hydrogen pro- duction rates over laboratory prepared catalyst were found slightly higher (5%) than commercial BASF catalyst
![]()
Figure 16. Graph of lnk against 1/T from obtained data at the 1/8 EtOH/H2O ratio with 5 grammes of commercial BASF catalyst [48] .
![]()
Figure 17. Lnk graph against 1/T from obtained data at the 1/8 EtOH/H2O ratio with 5 grammes of laboratory prepared catalyst [48] .
at temperatures between 700˚C - 900˚C and activity of laboratory prepared cataliyst is better than activity of commercial BASF catalyst at temperatures above 700˚C. Because, differences in hydrogen production rates were less even at temperatures above 700˚C for a duration of 2 weeks. But, differences in hydrogen production rates were higher (10% - 15%) over commercial BASF catalyst above 700˚C. This means that activity of com- mercial BASF catalyst was reducing at temperatures above 700˚C.
In this study, it can be seen that overall reaction is an endothermic reaction. The lower values of activation energies demonstrate that diffusion resistance has not an important effect on reaction rate. Catalyst loads were kept lower (1 to 5 grammes) in order to provide isothermal temperature distribution in the reactor content. Thus, measurements of intrinsic reaction kinetic were purposed. Experimental studies with different catalyst dimen- sions and different time on steams (0 - 240 minutes) were conducted at the temperature range of 250˚C - 700˚C and different ethanol/water ratios. The activation energy values of reaction were in the range of 6.504 kJ/mole to 52.717 kJ/mole, and the average value of activation energy was calculated as 26.870 kJ/mole. The reaction was controlled by chemical reaction, but in the case of higher activation energies, some diffusion resistances were taken place in the catalyst bed. The obtained activation energy values which are identical with the activation energies with similar catalyst contents have been presented in literatüre [50]-[52]. The sintering ratio of com- mercial BASF catalyst was found higher at operational temperatures over 700˚C than sintering ratio of labo- ratory prepared catalyst at similar operational conditions. The optimum life time on stream of commercial BASF catalyst was found as one week, and the optimum life time on stream of laboratory prepared catalyst was found as 2 weeks at operational temperatures up to 900˚C.
As a result of experimental measurements and some calculations, we have found that by reducing ethanol ratios in the water is resulting higher hiydrogen production rates at the exit of reactors. In the case of higher ethanol/water ratios in the entrance of the reactor, lower hydrogen yields were detected at theexit of the reactors. In the case of higher ethanol/water ratios in the feed, higher coke formation rates have obtained on the surface of the catalyst. Therefor, lower ethanol/water ratios were prefered during this experimental study, and so, higher hydrogen production rates had determined at the exit of the experimental system. We are planning to do some further experimental measurements related to this sutdy over various catalyst contaminations at a temperature range of 200˚C - 900˚C. By performing such an experimental programme, more valuable data will be produced for industrial hydrogen productions.
Acknowldegements
Authors wishes to thank Chemical Engineering Department of Atatürk University, for the given opportunity to work in their Laboratory during experimental measurements. Authors also thanks to Turkish Scientific Research Council (TÜBİTAK) and Atatürk University for financial supports during project studies.