New Sulfonamides Derived from Carvacrol: Compounds with High Antibacterial Activity against Resistant Staphylococcus aureus Strains ()
Received 27 May 2016; accepted 17 July 2016; published 20 July 2016

1. Introduction
Bacterial antibiotic resistance is an increasing reality in modern medicine and it is emerging as a significant threat to public health [1] [2] . In general, bacteria may have intrinsic or innate resistance to drugs or the ability to acquire said resistance [3] . Moreover, the accelerated evolution and spread of antibiotic resistant bacteria has been impelled by the widespread overuse of antibiotics within human medicine and outside of human medicine in agriculture and aquaculture [4] .
Resistance to the drugs used as therapeutic agents is a major concern, mainly in hospitals, because of the number of more advanced medical procedures, patients with suppressed immunity and due to new bacterial strains, which are multi-resistant [5] [6] . The major pathogen related to serious hospital infections is Staphylococcus aureus with its feature of gaining antibiotic resistance easier than other pathogens [7] .
Staphylococcus aureus is a gram-positive bacterium residing on the skin surface and mucous membranes of warm-blooded animals [8] . This pathogen causes a broad spectrum of infections, including wound infections, food poisoning, toxic-shock syndrome, osteomyelitis, pneumonia, brain abscesses, meningitis, bacteremia and various other conditions [9] . Furthermore, S. aureus has developed a stress response mechanism which allows it to adapt to the host. This survival ability under stressed circumstances, such as antibiotics and biological bactericidal factors, is related to bacterial gene mutation and horizontal transfer of resistant genes from external sources [10] .
The drug resistance spectrum of S. aureus includes the principal classes of antibiotics in use or that have been used as penicillin, methicillin, fluoroquinolones and vancomycin [11] . Organisms that are resistant to all approved antibiotics involve treatment with experimental and potentially toxic drugs [12] . Consequently, it is necessary to discover new and more effective antibacterial agents to treat infections caused by antibiotic resistant bacteria.
Sulfonamide moiety, -SO2NH-, occurs in a significant number of bioactive compounds with several activities including antibacterial [13] [14] , antitumor [15] , anti-carbon anhydrase [16] , antidiabetic [17] , hypoglycemic [18] , diuretic [19] , antithyroid [20] , protease inhibitory activity [21] [22] and analgesic [23] among others. Sulfonamides were the pioneers among the chemotherapeutic agents systematically used for the prevention and cure against various diseases [24] . The low cost and low toxicity associated with this class of molecules makes them valuable for application in the synthesis of derivatives [25] .
In this context, carvacrol (2-methyl-5-isopropyl-phenol) is an interesting basic fragment to introduce substituents. This monoterpenic phenol is the main constituent of essential oils of various aromatic plants such as Origanum, Thymbra, Thymus, Satureja, Coridothymus of the Lamiaceae family and Lippia of the Verbenaceae family, among others [26] . The molecule had showed a wide spectrum of antibacterial activity acting against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Lactobacillus plantarum, Pseudomonas fluorescens, Serratia spp., Enterobacter spp., Klebsiella pneumonia, Proteus mirabilis, Staphylococcus epidermidis and Streptococcus pneumoniae [27] [28] . Furthermore, carvacrol is characterized by having several other biological properties such as antifungal activity [29] [30] , anti-inflammatory [31] [32] , antioxidant [33] , antimutagenic [34] , AChE inhibitory [35] , analgesic [36] , antiparasitic [37] [38] and antimicrobial [39] [40] .
Research for chemically modified molecules seeks to achieve better biological properties, such as prolonged action, more effective antibacterial activity or wider spectrum of microorganism.
In this study we report the synthesis of sulfonamides derived from carvacrol, unprecedented in the literature, with excellent results for the antibacterial activity investigated.
2. Results and Discussion
2.1. Synthesis of Sulfonamides (Sulf-1 to Sulf-9)
The synthesis of new sulfonamides (SULF-1 to SULF-9) was performed in two steps: firstly the synthesis of 4-hydroxy-2-isopropyl-5-methylbenzene-1-sulfonyl chloride (ChS) was performed, subsequently, the ChS was used in reactions with various amines (Scheme 1), in good yields (Table 1).
ChS was obtained from the reaction of carvacrol to six equivalents of chlorosulfonic acid. Excess acid in reactions of chlorosulfonation of aromatic systems has been previously studied and it was also verified that the excess is the optimal amount for obtaining the best yields [41] . ChS is a difficult compound to characterize because it is a very unstable (it contains chlorine, which is a good leaving group and can be easily exchanged for
![]()
Table 1. Physico-chemical data for sulfonamides SULF-1 to SULF-9.
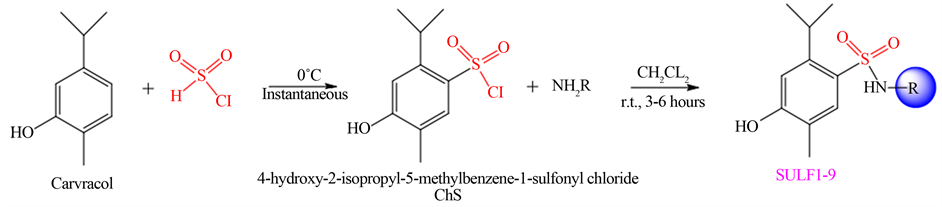
Scheme 1. Synthesis of sulfonyl chloride and sulfonamides SULF-1 to SULF-9.
water or other nucleophilic species). This compound undergoes hydrolysis and easily decomposes, so it needs special care for storage.
The sulfonamides obtained in this study were prepared from ChS with two equivalents of amine added slowly. The excess amine is also intended to neutralize the HCl formed during the reaction. Low temperature is recommended when amines are reactive. All sulfonamides synthesized in this work are unpublished in the literature.
2.2. Antibacterial Activity
The investigation of possible products of natural origin that might act as antimicrobial agents has been the subject of worldwide study, mainly against microorganisms that present resistance to commonly used antimicrobials.
Preliminary studies showed that carvacrol presented potential antimicrobial activity [42] - [46] prompting the interest in the study of this compound in terms of its antibacterial potential in resistance. Carvacrol and its derivatives were tested to determine their capacity to inhibit the growth (MIC) of six multi-drug resistant strains, methicillin-resistant Staphylococcus aureus (Sa 1 to Sa 8) and one standard stain S. aureus through the microdilution method.
As can be observed in Table 2, carvacrol exhibited activity against the standard strain S. aureus tested (ATCC 25923), with an MIC equivalent of 390 ppm, but its activity was weak against resistant strains. However, the nine derivatives tested showed much better activity against all strains tested.
The most active compounds were Sulf-1, Sulf-3 and Sulf-8, in this order, with MIC values ranging from 3.90 to 62.50 ppm. From these, compound Sulf-1 was the one that showed the best performance for all tested strains of bacteria (MIC = 3.90 to 15.62 ppm). The results demonstrated that carvacrol derivatives present excellent activity and showed the potential of this compound as a possible antibacterial agent to treat infectious processes caused by S. aureus.
2.3. Evaluation of Synergistic Effect
Currently, novel antimicrobials cannot replace antibiotics, but they may become valuable antibiotic complements.
Since the compounds Sulf-1, Sulf-3 and carvacrol showed activity against the bacteria S. aureus, further studies were conducted to verify the possible synergistic action between the compounds and antibiotics. Synergism is a type of biological response obtained from the association of two or more antimicrobials whose resultant is greater than the simple sum of the single effects. Synergism may occur between drugs and is a novel concept that could be beneficial (synergistic or additive interaction) or deleterious (toxic or antagonistic outcome) [47] [48] .
The Fractional Inhibitory Concentration index (FICI), represents the sum of the FIC of each drug tested (SULF or antibiotic), where the FIC for each drug is determined by dividing the MIC when used in combination, by the MIC when used alone. These results can be used to determine synergistic and antagonistic interactions.
The results of the combinations are shown in Table 3, and as can be seen, the combinations between the compound (Sulf-1, Sulf-3 or carvacrol) against each antibiotics (amoxicillin, ampicillin, erythromycin and tetracycline)
![]()
Table 2. Minimum inhibitory concentration for sulfonamides Sulf-1 to Sulf-9, against different strain of Staphylococcus aureus.
![]()
Table 3. Avaliation of synergistic effect between SULF-1, 3-SULF and carvacrol with different antibiotics.
aMIC of antibiotics in combination with SULF1, SULF3, carvacrol, and in parentheses MIC of antibioticsin combination with SULF (1 or 3) or carvacrol. bFICof antibiotics in combination with SULF1, SULF3, carvacrol, and inparentheses the FIC of SULF or Carvacrol.
exhibited an FICI between 0.37 to 2.0. Carvacrol exhibited a type of interaction classified as interaction indifferent (FICI between 1 and 4). The same kind interaction was found for compound SULF-1 with amoxicillin, ampicillin and tetracycline with FICI values equal or greater than 1.0, however, an important synergistic effect was found when in combination with erythromycin (FICI 0.37).
The compound SULF-3 also presented a synergistic effect when combined with tetracycline (FICI 0.50) and partial synergistic effect when combined with ampicillin (FICI 0.75).
An alternative approach to improve the survival of patients with infections which do not respond well to single- drug therapy due to lack of efficacy or rapid emergence of resistance, could be to combine antibacterial agents. Combination therapy has been shown to be beneficial for several difficult-to-treat infections.
3. Conclusions
A series of nine sulphonamide derivatives of carvacrol were synthesized. These compounds, after purification and characterization, were evaluated for antibacterial activity. The results showed high antibacterial activity for 8 resistant S. aureus strains, especially the compounds Sulf-1, Sulf-3 and Sulf-8, which showed the best results, even better results than carvacrol, composed of recognized antibacterial activity.
Based on the FICI values, the combinations of SULF-1 and SULF-3 with antibiotics, showed a synergistic effect with an FICI of 0.5 or less against the reference strain of S. aureus, results which can be considered very promising. This encourages new tests to verify the applicability of these compounds in the therapy of infectious processes of difficult treatment today.
4. Experimental
4.1. Synthesis of Sulfonamides SULF-1 to SULF-9
All the solvents used were analytically pure. The reagents 5-isopropyl-2-methylphenol (carvacrol), 4-methylani- line, 4-nitroaniline, 4-fluoroaniline, 2-hydroxyaniline, 4-methoxyaniline, naphthalene-2-amine, 2-aminopyrazine, sulfathiazole, 2,4-dinitrophenyl hydrazine and chlorosulfonic acid were obtained from Sigma Aldrich.
The compounds obtained were analyzed by thin layer chromatography (TLC) using aluminum plates coated with silica gel 60 GF254 (Merck). Melting points were recorded on the apparatus PF Chemistry MQA. 1H and spectra were obtained using a Varian AS-400 spectrometer operating at 400 MHz. The mass analysis was performed on an amaZon speed ETD Trap Mass spectrometer.
4.1.1. Procedure for the Preparation of Sulfonamides SULF-1 to SULF-9
The 4-hydroxy-2-isopropyl-5-methylbenzene-1-sulfonyl chloride (or simply ChS) was obtained from the reaction of carvacrol with 6 equivalents of chlorosulfonic acid. The chlorosulfonic acid was cooled in an ice bath for 15 minutes and, after, carvacrol was carefully dripped. The product was poured on ice and purified.
The sulfonamides SULF-1 to SULF-9, were prepared from one equivalent of ChS and two equivalents of different amines. The reaction was carried out using dichloromethane as a solvent. The reactions were followed by TLC. After the formation of the products, the dichloromethane was evaporated and the products were purified by acid-base extraction.
4.1.2. Spectral Characterization and Mass Analysis of SULF-1 to SULF-9
4-hydroxy-2-isopropyl-5-methyl-N-(p-tolyl)benzenesulfonamide (SULF-1):
Yellowish solid (m.p 162˚C - 164˚C). 1H NMR (deuterated acetone): 6.95 to 7.73 (6H, multiplet, H aromatic); 3.82 (1H, quintet, H Alifatic.); 2.12 (3H, singlet, methyl); 1.15 (3H, singlet, methyl); 1.13 (3H, singlet, methyl). MS (m/z, M-H): 318.12.
4-hydroxy-2-isopropyl-5-methyl-N-(4-nitrophenyl)benzenesulfonamide (SULF-2):
Yellow solid (m.p 179˚C - 180˚C). 1H NMR (deuterated acetone): 6.67 to 7.65 (6H, multiplet, H aromatic); 3.81 (1H, quintet, H Alifatic.); 2.12 (3H, singlet, methyl); 1.16 (3H, singlet, methyl); 1.13 (3H, singlet, methyl). MS (m/z, M-H): 349.15.
N-(4-fluorophenyl)-4-hydroxy-2-isopropyl-5-methylbenzenesulfonamide (SULF-3):
Lilac solid (m.p 181˚C - 183˚C). 1H NMR (deuterated acetone): 6.72 to 6.93 (6H, multiplet, H aromatic); 3.81 (1H, quintet, H Alifatic.); 2.15 (3H, singlet, methyl); 1.14 (3H, singlet, methyl); 1.11 (3H, singlet, methyl). MS (m/z, M-H): 322.33.
4-hydroxy-N-(2-hydroxyphenyl)-2-isopropyl-5-methylbenzenesulfonamide (SULF-4):
Brown solid (m. P. 152˚C - 153˚C). 1H NMR (deuterated acetone): 6.95 to 7.69 (7H, m, H aromatic); 3.82 (1H, quintet, H Alif.); 2.16 (3H, singlet, methyl); 1.16 (3H, singlet, methyl); 1.13 (3H, singlet, methyl). MS (m/z, M + H): 322.1015.
4-hydroxy-2-isopropyl-N-(4-methoxyphenyl)-5-methylbenzenesulfonamide (SULF-5):
Yellow solid (m.p 172˚C - 173˚C). 1H NMR (deuterated acetone): 6.73 to 7.64 (6H, multiplet, H aromatic); 3.81 (1H, quintet, H Alifatic.); 3.70 (1H, singlet, OCH3); 2.11 (3H, singlet, methyl); 1.16 (3H, singlet, methyl); 1.13 (3H, singlet, methyl). MS (m/z, M-H): 349.15. MS (m/z, M-H): 334.12.
4-hydroxy-2-isopropyl-5-methyl-N-(naphthalen-2-yl)benzenesulfonamide (SULF-6):
Pink solid (m.p. 201˚C - 103˚C). 1 H NMR (deuterated acetone): 6.76 to 7.80 (9H, multiplet, H aromatic); 3.77 (1H, quintet, H Alifatic); 2.16 (3H, singlet, methyl); 1.19 (3H, singlet, methyl); 1.15 (3H, singlet, methyl). MS (m/z, M + H): 356.08.
4-hydroxy-2-isopropyl-5-methyl-N-(pyrazin-2-yl)benzenesulfonamide (SULF-7):
White solid (m.p. 165˚C - 167˚C). 1H NMR (deuterated acetone): 7.65 (1H, singlet, H aromatic); 7.30 (3H, multiplet, aromatic H); 6.97 (1H, singlet, aromatic H); 3.83 (1H, quintet, H Alifatic); 2.20 (3H, singlet, methyl); 1.20 (3H, singlet, methyl); 1.17 (3H, singlet, methyl). MS (m/z, M + H): 308.23.
4-hydroxy-2-isopropyl-5-methyl-N-(4-(N-(thiazol-2-yl)sulfamoyl)phenyl) benzenesulfonamide (SULF-8):
Beige solid (m.p. 198˚C - 199˚C). 1H NMR (deuterated acetone): 7.73 to 7.81 (8H, multiplet, H aromatic); 3.83 (1H, quintet, H Alifatic); 2.19 (3H, singlet, methyl); 1.14 (3H, singlet, methyl); 1.10 (3H, singlet, methyl). MS (m/z, M + H): 468.06.
N'-(2,4-dinitrophenyl)-4-hydroxy-2-isopropyl-5-methylbenzenesulfonohydrazide (SULF-9):
Red solid (m.p. 212˚C - 113˚C). 1H NMR (deuterated methanol): 6.93 to 9.09 (5H, multiplet, H aromatic); 3.66 (1H, quintet, H Aliphatic); 2.20 (3H, singlet, methyl); 1.24 (3H, singlet, methyl); 1.21 (3H, singlet, methyl). MS (m/z, M + H): 411.12.
4.2. Microorganisms
For the antimicrobial evaluation the Staphylococccus aureus (ATCC 25913) strain of the American Type Culture Collection, Rockville, MD, USA, was used and the methicillin-resistant S. aureus (MRSA) strains (Sa 1, Sa 2, Sa 3, Sa 5, Sa 6, Sa 8) were obtained from clinical material collected at a hospital as part of a study conducted in 2005 and 2006 (unpublished data), in Itajaí, Santa Catarina, Brazil. All staphylococcal strains tested were identified by standard methods (colony morphology, Gram staining, catalase testing, tube coagulase testing, and by testing for DNase activity) and agar screening method for antimicrobials susceptibility. The strains were deposited at the Laboratory of Research in Microbiology at the University of Univali in Itajaí, Santa Catarina, Brazil.
4.3. Antimicrobial Susceptibility Testing (AST)
The Antimicrobial susceptibility of different S. aureus strains were evaluated by disk diffusion, using Mueller- Hinton agar plates inoculated with a 0.5 McFarland standard, according to CLSI (2012) recommendations. The disks were applied and after incubation overnight at 35˚C, zone diameters were read with the aid of calipers.
4.4. Determination of MIC
Antibacterial susceptibility testing of the compounds against resistant S. aureus strains (Sa 1, Sa 2, Sa 3, Sa 5, Sa 6, Sa 8), was determined by the broth microdilution method recommended by the CLSI M7-A8 (CLSI, 2009). For minimum inhibitory concentration determinations (MIC), suspensions with 0.5 McFarland turbidity equivalence standards were prepared by suspending growth from Mueller-Hinton agar plates in 2 mL of sterile saline. The suspensions were further diluted 1:10 to obtain a final inoculum of 105 CFU/mL, and a range of compound concentrations (1000 to 2 ppm or 64 to 0.125 ppm to vancomycin) were used. Microtiter plates were incubated at 35˚C and the MICs were recorded after 24 hours of incubation. The MIC was defined as the lowest concentration of compounds in which the microorganism tested did not demonstrate visible growth. A standard quality control strain (S. aureus ATCC 25913) and the antibiotic vancomycin (Sigma V2002) were included in each run.
4.5. Synergism Testing (Checkerboard Method)
The synergism test was conducted for the compounds Sulf-1, Sulf-3 and carvacrol, since they showed the best results of MIC. The microdilution checkerboard method is the technique used most frequently to assess antimicrobial combinations in vitro. Dilutions of compounds Sulf-1, Sulf-3 and carvacrol were made for the evaluation of the interactions. The type of interaction studied was on the S. aureus strain. Dilutions from the logarithmic- growth phase of the bacterial culture were prepared and distributed into microtiter trays containing varying concentrations of the different compounds.
Synergy testing was performed with Staphylococccus aureus strains (ATCC 25913) by checkerboard titration in microtiter plates with twofold serial broth microdilutions [48] . Sulf-1 was tested at 15.62 to 0.24 ppm, Sulf-3 was tested at 31.25 to 0.48 ppm and carvacrol at 781.25 to 12.20 ppm (two times the MIC). Wells were assessed visually for growth after an 18 hour incubation period at 37˚C. The fractional inhibitory concentration (FIC) was calculated for each inhibitor and antibiotic (amoxicillin, ampicillin, erythromycin and tetracycline) combination. The following formulas were used to calculate the FIC index: FIC of drug A = MIC of drug A in combination/MIC of drug A alone, FIC of drug B = MIC of drug B in combination/MIC of drug B alone, and fractional inhibitory concentration index (FICI) = FIC of drug A + FIC of drug B. Synergy was defined as an FICI of <0.5. FICI values between 0.5 - 0.75 denoting partial synergy; 0.76 - 1.0 denoting additive effect; 1 - 4 denoting indifference; and >4 denoting antagonism.
Acknowledgements
The authors thank CNPq for financial support and CEBIME for mass analysis.