Modeling Operational Parameters for Uranium Dioxide Production Reactor through Uranium Trioxide Reaction Using Hydrogen ()
Received 4 May 2016; accepted 6 July 2016; published 9 July 2016

1. Introduction
Nuclear energy is a source of major electricity production, due to the high-energy capacity of the uranium fuel element during nuclear fission. Uranium is used mainly in the form of UO2 type compounds. For this purpose, it must be enriched in its U235 isotope to fulfill this purpose. The needed enrichment level will depend on the type of reactor used, and there are 2 types: Light Water Reactors (LWR), which require an enrichment of U235 minimum between 3 and 5% to operate, and Pressurized Heavy Water Reactor (PHWR), which uses natural uranium as fuel [1] .
Figure 1 shows the uranium conversion for the production of UF6, raw material for the enrichment process.
This process reduces the uranium compound, either from UO3 or from U3O8, and obtains UO2 powders, according to the overall reaction:
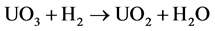 . (1)
. (1)
Subsequently, UO2 powder should be treated by hydrofluorination to obtain UF4 powders:
 . (2)
. (2)
Finally, the UF4 compound is taken to the fluorination process to obtain UF6:
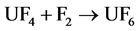 . (3)
. (3)
The UF6 compound, gaseous at 56˚C and atmospheric pressure, is carried to the enrichment process, in order to obtain a concentrated and a diluted fraction in U235 through centrifugal force reactors [3] , using the different weights of this and the U238 isotope. This process is shown schematically in Figure 2 [4] .
The gaseous compound UF6, once enriched, must return to its UO2 form. To do this, there are 3 methods to carry out this task [7] [8] .
H2 reduction: The UF6(g) compound is reduced using hydrogen according to the reactions:
 (4)
(4)
 . (5)
. (5)
Hydrolyzing: The UF6(g) compound is hydrolyzed using water:
 (6)
(6)
 . (7)
. (7)
Subsequently, the ammonium diuranate is reduced using H2 at temperatures of 600˚C - 800˚C:
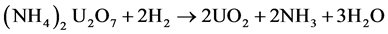 . (8)
. (8)
Finally, the third method for enriched UO2 production is through precipitation of ammonium uranyl carbonate instead of ammonium diuranate, according to reaction (10):
![]()
Figure 1. Uranium conversion process [2] [3] .
![]()
Figure 2. The process leading to obtain UO2 fuel elements [5] [6] .
 . (10)
. (10)
All these processes require the use of a fluidized bed reactor for producing UO2 for both UF6 production, intended for further uranium enrichment, as for reproduction as UO2 fuel pellets for nuclear power reactors.
It is for these reasons that this work will evaluate the main operating parameters of a fluidized bed reactor for UO2 production from UO3 concentrates using mathematical modeling techniques and the COMSOL Multiphysics software, in its 4.3b version. These parameters are the followings: feed flow, hydrogen concentration and initial temperature.
Conventional UO3 to UO2 reduction reactor is shown in Figure 3.
UO2 production is conventionally performed in a reduction reactor as shown in Figure 3. Here, uranium trioxide and hydrogen are continuously fed, H2 is normally diluted in an inert gas such as argon or nitrogen. It is also common to use ammonia, which is cracked [11] to obtain a nitrogen and hydrogen gas mixture. This UO2 production reactor [12] [13] is controlled by the following operational parameters: composition and flow of the reducing gas, UO3 feed and internal reactor temperature.
2. Theoretical Basis [14]
2.1. Momentum Transport
Momentum transport is given by the Navier-Stokes equation, for compressible fluids:
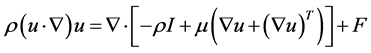 (11)
(11)
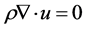 (12)
(12)
where: ρ: density, u: H2 flow velocity, I: identity matrix, F: External forces.
Momentum transport will determine the gas behavior inside the reduction reactor, for laminar regime.
2.2. Heat Transport
Heat transport is determined by the following equations:
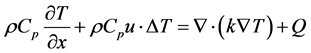 (13)
(13)
![]()
Figure 3. Conventional UO2 production reactor [9] [10] .
![]() (14)
(14)
where ρ: reduction gas density, Cp: specific heat, u: H2 flow velocity, T: temperature, k: conduction coefficient, Q: source term, t: time, v: volume.
Solving this equation in the model will determine the temperature inside the UO3 reduction reactor and the generated heat by the exothermic reaction between the UO3 concentrate and the H2 reducing gas.
2.3. Transport of Diluted Species
Mass transport for dilute species is determined by the equation:
![]() (15)
(15)
where D: Hydrogen diffusion coefficient, C: Hydrogen concentration in the reduction gas, u: Gas velocity flow, R: Hydrogen consumption rate in the reaction zone.
This equation will determine the concentration profile of hydrogen and/or water displayed by the system in continuous operation type.
3. Reactor Modeling [15]
The reactor used in the development of experiences is shown in Figure 4.
This reactor is a tubular type, with input and output in conical shape. Its length is 1.66 m . and it has an internal diameter of 4.5 cm . The development of the experiences was performed by setting as parameters the initial temperature of both the gas and the reactor, which was between 400˚C - 500˚C and initial hydrogen concentrations in the reducing gas: 0.25, 0.5 and 0.75 M . The flow feeding the reduction reactor was kept constant at 2.5 L/min. The input speed of this flow was 0.1 m /s, and the cross section of 1.5 cm, so the feed flow regime was laminar.
To develop this model, the 3 transport phenomena mentioned before were occupied: momentum, heat and mass for dilute species. Working conditions are shown in Figure 5:
Figure 5 shows the considered parameters for the modeling: In the case of momentum transport, the reducing gas has inlet and outlet inside the reactor, and there is no slip flow on the walls. For heat transport, it is considered that the initial temperature for uranium conversion processes is 700 K. The walls symbolize heat losses by natural convection, where this system was covered by a thermal jacket at 450 K. The remaining heat will be generated by the exothermic reaction inside the vessel between UO3 and H2. Finally, for the transport of diluted species, reducing gas flow has the aforementioned initial hydrogen concentrations. A fraction of this gas is consumed by the UO3 vessel. The remaining hydrogen at the reactor outlet was burned to prevent gas leakage. In
![]()
Figure 5. Working conditions for UO2 production.
order to simplify the development of the calculations, the model was proposed using as axial symmetry, in the bottom of Figure 5.
The equations used to the model development were obtained from previous studies [15] . In this work, the reduction kinetics of uranium trioxide was developed based on the formation of an intermediate compound, U3O8, according to the following reactions:
![]() (16)
(16)
![]() . (17)
. (17)
The reduction rates for both equations were determined by using Arrhenius’s Law, according to Equation (18):
![]() (18)
(18)
where![]() : UO3 reaction rate, cH2: hydrogen concentration in the reducing gas, n: reaction order for hydrogen concentration, R: universal gas constant, T: absolute temperature, Ea: activation energy.
: UO3 reaction rate, cH2: hydrogen concentration in the reducing gas, n: reaction order for hydrogen concentration, R: universal gas constant, T: absolute temperature, Ea: activation energy.
The corresponding reaction rates for Equations (17) and (18) are, respectively:
![]() (19)
(19)
![]() . (20)
. (20)
According to other authors [16] , the conversion process is usually made from U3O8 compounds, as the uranium oxide with greater chemical stability. This implies that reaction (19) occurs uneventfully. Moreover, reaction rate (20) involves the formation of other intermediate oxides of uranium, such as U3O7 or U4O9, which, as already discussed in other studies [15] [16] , decrease the overall speed of the process at temperatures above 700˚C. For this reason, reaction kinetics (21), which is the controlling step, will be used for modeling the reduction reactor.
4. Results
The obtained results were as follows:
According to Equations (12) and (13), it can be said that the progress of these reactions is quantified through water production. Figure 6 shows the UO3 reduction kinetics is so fast at these temperatures, almost no hydrogen exists in the vicinity of the vessel, where the reaction occurs. The following figure shows the linear profiles generating water into the reactor, along the axial symmetry.
Figure 7 shows that, for all hydrogen concentrations in the reducing gas, they are consumed almost entirely in the UO3 vessel.
However, the most important parameter for the development of experiences is the temperature profile along the reactor, and the exothermic peak reached by the system due to the release of reaction heat, as this defines the capacity of the system to work autonomously, without the need of additional energy. This profile is determined by the release of energy from the exothermic reaction between uranium oxides and hydrogen. The profiles obtained for the hydrogen concentrations in the reducing gas, are shown in Figure 8.
According to bibliographic data [15] , the ideal temperature for the development of these experiences is 500˚C - 600˚C. The objective of this result is to check whether the process can operate autonomously, keeping the temperature within this range. For this reason, the process must be performed at 0.5 M H2 concentrations to ensure this stationarity condition.
5. Validation of Results
The results of the experiments carried out in this reactor are shown in the following figure:
Figure 9 shows that UO3 reaction kinetics is virtually instantaneous in contact with the reducing H2 gas, since for temperatures of 500˚C and 600˚C, the transformed fraction of UO3 to UO2 it is above 90%.
In the case of temperature, the experimental exothermic peaks obtained are compared with the modeling system.
![]()
Figure 6. Volumetric concentration profile of water inside the reduction reactor.
![]()
Figure 7. Water profile concentrations inside the reduction reactor.
![]()
Figure 8. Temperature profile for the UO3 reduction reactor.
![]()
Figure 9. Reaction kinetics for uranium concentrates, at constant temperature.
![]()
Table 1. Comparison between exothermic peaks.
Table 1 shows that the temperature system, operating continuously, can be predicted in an acceptable way using mathematical modeling techniques, since the data obtained for the proposed working conditions show relative errors of less than 10%.
6. Conclusions
・ From the previous data of UO2 production, it is possible to predict the reduction of UO3 compounds with hydrogen using mathematical modeling techniques.
・ The fluidized bed reactors can be monitored effectively with the gaseous products, which means that it is not necessary to manipulate the UO3 vessel. This fact allows the safety during the data collection.
・ For the studied kinetic parameters, the UO3 reduction reactions occur almost instantly, as the results show a hydrogen conversion to water almost completely.
・ The condition that allows continuous operation for the reduction reactor is feeding with reducing gas at 0.5M H2.
・ The temperature profiles and relative errors allowed to conclude that the reduction of U3O8 compounds to UO2 provided the required energy to maintain the working temperature in the required ranges.
Acknowledgements
The Chilean Nuclear Energy Commission acknowledges the assistance provided by University of Santiago, Chile, in the use and license of computer software COMSOL Multiphysics v4.3b, “CFD Module User’s Guide”, Modeling Single Phase Flow, Page 27-144. License Number: 2079130, Host ID: 5c260a04780b, 1.998-2.103.