The Relationships between Dyeing Methods and Dyeability in Hair Colouring by Utilising Enzymatic Oxidation of (+)-Catechin ()
Received 23 March 2016; accepted 15 May 2016; published 18 May 2016

1. Introduction
The oxidation hair dyeing occasionally gives skin irritation and damages to human hair. The oxidation hair dye products contain some materials working as strong allergens and causing sensitisation symptoms during the dyeing process [1] - [4] . Under such the situation, the last author’s research group has been studying novel hair dyeing techniques by using bio-based materials obtained from plants in order to invent safer ones. The (+)-cate- chin ((+)-(2R,3S)-5,7,3’,4’-tetrahydroxyflavan-3-ol; Cat Figure 1(a)) is one of the flavonoids extracted from tea plants, gambir (Uncaria gambir), areca (Areca catechu) and so on. The Cat shows reductive action and its catechol group forms complexes with metal ions [5] [6] .
It was found that the oxidation product of Cat, which was named catechinone, works as hair dyestuff and the dyestuff does not cause erythema or oedema on skin of rabbits [7] . The main compound of catechinone is 4-(3, 4-dihydro-3α,5,7-trihydroxy-2H-1-benzopyran-2α-yl)-1,2-benzoquinone (Figure 1(b)) and the dyestuff contains a small amount of chromatic side products [7] . The colour of powder and solution of catechinone are red, and the absorption peak and the strength change with solution pH and concentration of added salts.
The catechinone dyes hair orange, reddish orange or deep yellowish brown depending on dyeing conditions such as the oxidation method, dye concentration, temperature, pH and additives [7] [8] . The colour fastness of the hair dyed by catechinone to washing and light (daylight and ultraviolet light) is high enough as same as that of oxidation hair dyes [7] [8] . However, the dyeability of catechinone is not high enough for practical use, because the hair dyeing by using it is mild way and the dye colourant molecules do not vigorously penetrate into hair. Therefore, improving its dyeability without using harmful agents is important and requisite for developing the technique.
The catechinone is obtained by the enzymatic [7] [9] or chemical [10] [11] treatment of Cat, and the oxidation reactions are occurred at the catechol (o-dihydroxybenzene) group of Cat to give a corresponding o-quinone. The enzymatic technique shows generally the advantage in the reactivity and selectivity. In the enzymatic way, catechinone is produced from Cat aqueous solution with copper-containing enzyme such as tyrosinase and L- ascorbate oxidase [7] . The enzymatic reaction between (+)-catechin and tyrosinase requires a large amount of oxygen and consumes rapidly oxygen dissolved in a reaction solution in a few minutes [9] . Continuous introduction of oxygen gas into the reaction solution is needed for the efficient production of catechinone by the enzymatic reaction. The optimum conditions for the production of catechinone are as follows: the higher concentration of dissolved oxygen (≥1 mM in water), 30˚C of reaction temperature, 8.4 - 9.0 of the solution pH [9] .
The improvement of the dyeability of the hair colouring by catechinone is indispensable as mentioned above. In the research, the relationships of the Cat oxidation and dyeing conditions as dyeing methods to the dyeability of hair in the hair colouring by catechinone were studied. The four kinds of dyeing methods are as follows:
1) Redissolution Dyeing Method: dyeing hair in the dye solution containing redissolved catechinone, which is preliminarily obtained by the enzymatic oxidation of Cat.
2) Sequential Dyeing Method: dyeing hair in the dye solution just after the catechinone being formed by the enzymatic reaction in it.
3) Simultaneous Oxidation Dyeing Method: dyeing hair in solution containing Cat and the dyeing process is accompanied by the enzymatic oxidation of Cat.
4) Post-Oxidation Dyeing Method: hair is immersed into Cat solution at the first step and then the hair is treated in another solution containing enzyme at the second step.
![]()
![]() (a) (b)
(a) (b)
Figure 1. Chemical structure of (+)-catechin ((a) Cat) and catechinone (b).
The enzyme used in the study was tyrosinase obtained from mushroom. The effect of temperature in the oxidation and dyeing process on the dyeability of hair was also studied.
2. Experimental
2.1. Materials
(+)-Catechin (Cat, Mw = 290.27, Sigma) was used as the dyestuff precursors without further purification. Tyrosinase (Mw = 1.28 × 105 [sedimentation velocity diffusion], 1.33 × 105 [light-scattering measurements], 1.20 × 105 [electrophoresis], Sigma) was used as received. Disodium hydrogen phosphate (Na2HPO4, Fw = 141.96, Nacalai Tesque) and sodium dihydrogen phosphate (NaH2PO4, Fw = 119.98, Nacalai Tesque) as pH regulators were used without further purification. Water was used as the solvent after distillation.
The human hair samples (Mathai Japan, obtained from Asians and decolourised white, length: ca. 11 cm) were bundled by a nylon band and kept under a low humidity. NLES-227 (Taiko Oil Chemicals) that contains 27 wt% of sodium dodecyloxypolyoxyethylene sulphate (C12H25O∙(CH2CH2O)n∙SO3Na, average n is 2) was used as anionic detergent for washing hair.
2.2. Dyestuff Preparation by Enzymatic Oxidation
Catechinone was obtained enzymatically from Cat as reported previously [8] [9] . The dyestuff preparation from 5 mM of Cat was started by the addition of 64 U∙ml−1 of tyrosinase in 500 ml of phosphate buffer aqueous solution (0.1 M NaH2PO4/Na2HPO4, pH = 7.0), which was saturated with oxygen by introducing O2 gas (≥99.5 vol%) at 30˚C. The obtained solution was concentrated to be powdered.
2.3. Hair Dyeing
Dyeing hair was made by four kinds of procedures as follows:
1) Redissolution Dyeing Method
The white hair tress (0.8 g) was immersed into 0.29 wt% of the catechinone phosphate buffer aqueous solution (pH = 7.0, 100 ml).The catechinone was obtained as described at §2.2. The solution was shaken at 100 rpm of shaking speed for 40 min at 30˚C.
2) Sequential Dyeing Method
The Cat (10 mM) and tyrosinase (70 U∙ml−1) were dissolved into phosphate buffer solution (pH = 7.0) and were allowed to stand at 30˚C for 40 min with O2 gas being introduced at 100 ml∙min−1 of the rate. The hair sample was immersed into the dye solution with O2 gas being introduced at 30˚C for 40 min. The dyeing solution was shaken as the same way of 1).
3) Simultaneous Oxidation Dyeing Method
The Cat (10 mM) and tyrosinase (70 U∙ml−1) were dissolved into phosphate buffer solution (pH = 7.0, O2 had been saturated) and the hair sample was immersed into the solution. O2 gas was introduced into the shaken solution at 30˚C for 40 min.
4) Post-Oxidation Dyeing Method
The hair sample was immersed into 10 mM of Cat solution (pH = 7.0) at 30˚C for 40 min and the sample was then dipped into 70 U∙ml−1 of tyrosinase solution (pH = 7.0, O2 had been saturated) at 30˚C for 40 min with O2 gas being introduced. Both of the treatment solutions were shaken.
The hair samples were taken out from the solution immediately after each the treatment, washed with 100 ml of 0.8 wt% sodium dodecyloxypolyoxyethylene sulphate aqueous solution and rinsed twice with 200 ml of distilled water at 35˚C. The samples were air-dried at room temperature.
2.4. Measurements
The colour and K/S spectrum of hair were measured by a Konica Minolta CM-2600d spectrocolourimeteremploying 10˚-view angle, CIE standard illuminant D65 and SCI (specular component included) mode. All the reflected light from a sample including the regular reflection is integrated under the SCI mode. The resulting colour was expressed in L*a*b* standard colourimetric system (CIE 1976). The L* is the lightness index, and a* and b* are the chromaticity coordinates. The positive values of a* indicate red colours and the negative values of that indicate green ones, and the positive values of b* indicate yellow and the negative values indicate blue. The C* is the chroma calculated by C* = {(a*)2 + (b*)2}1/2. The K/S value is defined Kubelka-Munk theory [12] [13] and obtained from K/S = (1 − Rλ)2/2Rλ, where K is the absorption coefficient, S the scattering coefficient and Rλ the reflectance of the light (wavelength: λ) for measured samples. The used human hair is unevenly coloured. Then, the measurements of hair was conducted three times for each three parts (measured area: 3 mmφ), and they were averaged. The standard deviation of Rλ is within 0.1% - 0.2% and that of colour difference (ΔE* = {(ΔL*)2 + (Δa*)2 + (Δb*)2}1/2) is within 0.04. There is individual specificity for each the hair tresses, whereas the tendencies of the relationships between dyeing conditions and dyeability are almost constant.
3. Results and Discussion
3.1. Relationships between Dyeing Methods and Dyeability
The photographs of undyed hair sample and dyed one by each of the technique: 1) Redissolution; 2) Sequential; 3) Simultaneous Oxidation or 4) Post-Oxidation Dyeing Method using Cat are shown in Figure 2. The treatments were performed with or without O2 gas introduction for the 2) Sequential and 3) Simultaneous Oxidation Dyeing Method in order to observe the effect of oxygen on the dyeability. The dyed hair is pale yellow obtained by the Redissolution Dyeing Method and deeper yellowish brown by the Sequential and Simultaneous Oxidation Dyeing Method. The Sequential and Simultaneous Dyeing Methods with O2 gas give deeper colour of hair as compared with the methods without O2. The most reddish colour of hair is obtained by the 4) Post-Oxidation Dyeing Method as slightly reddish brown. The results of the colour and K/S measurements for the undyed and dyed hair samples are shown in Figure 3, Figure 4 and Table 1. The hair dyed by Post-Oxidation Method with O2 shows highest a* and lowest L* values and that by Simultaneous Oxidation Method with O2 shows highest b* value. The K/S value at 400 nm for the hair dyed by Simultaneous Oxidation Methods with O2 is highest and that at 550 nm by Post-Oxidation Method with O2 is highest.
The undyed hair shows very slightly yellow and exhibits lowest K/S spectrum as shown in Figure 4. The spectra of the dyed hair exhibit higher K/S values under 600 nm of λ, especially under 500 nm as compared with those of undyed one. The samples (Figure 2(c), Figure 2(d) and Figure 2(f)) exhibiting higher b* show higher K/S values at 500 nm and under, and the samples (Figure 2(c), Figure 2(d), Figure 2(f) and Figure 2(g)) exhibiting higher a* show higher K/S values 500 nm and over. In particular, the K/S spectrum for Post-Oxidation Method is highest 500 nm and over.
The dye formation reaction effectively proceeds by a large supply of O2 gas and this contributes the higher dyeability for the Sequential and Simultaneous Methods with O2 gas. The O2 gas supply is essential for proceeding the enzymatic reaction cycle, thus, the rate of dyestuff formation increases by oxygen introduction. In the case of Simultaneous Oxidation Method without O2 gas introduction, oxygen residue in the solution is consumed immediately and the dye formation reaction proceeds slowly. It results in the lower dyeability. In the case of Sequential Method without O2 gas introduction, its reaction time is twice as long as that of Simultaneous Oxidation Method. Therefore, a little bit more dyestuff is produced to give deeper colour to hair as compared
![]()
![]() (a) (b)
(a) (b)
Figure 3. Colour measurement results of hair samples undyed ( ) and dyed by redissolution dyeing method with catechinone with O2 gas introduced (
), sequential dyeing method without ( ) and with ( ) O2 gas, simultaneous oxidation dyeing method without ( ) and with ( ) O2 gas, and post-oxidation dyeing method with O2 gas ( ) by using (+)-catechin at 30˚C. (a) Chromaticity coordinates relationship (a*-b*), (b) chroma-lightness index relationship (C*-L*).
![]()
Figure 4. K/S spectra for the hair undyed (---) and dyed by redissolution Dyeing Method with catechinone (―), Sequential dyeing method without (- - -) and with (―) O2 gas introduced, simultaneous oxidation method without (---) and with (―) O2 gas introduced, and post-oxidation dyeing method (―) by using (+)-catechin at 30˚C.
![]()
Table 1. The colourimetric values and K/S ones for undyed and dyed hairs.
a Colour difference between the dyed hair and undyed hair; ΔE* = {(L* − L*0)2 + (a* − a*0)2 + (b* − b*0)2}1/2, which L*, a*, b* for the dyed hair and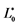 ,
, 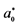 ,
, ![]() for the undyed hair.
for the undyed hair.
with that in the case of Simultaneous Oxidation Method without O2.
The dyeability of Simultaneous Oxidation Method with O2 gas is higher than that of Sequential Method with O2 gas, in spite of the longer oxidation reaction time in Sequential Method. Not only the catechinone is produced, but also dimers and multimers form in the solution during the oxidation of Cat [14] [15] . The reactive o-benzoquinone part of catechinone reacts with a raw Cat. The freshly-produced catechinone is consumed to give the dimers and multimers via some reaction routes during the reaction and their amount may increase with time. This may result in the lower dyeability in Sequential Method. The more freshly-produced catechinone in Simultaneous Oxidation Method contributes to the higher dyeability. This explanation is also supported by the fact that Redissolution Method shows lower dyeability and the dyestuff powder used in the method can contain the more dimers and multimers.
Cat molecules are absorbed into and adsorbed onto hair before oxidation process in Post-Oxidation Method, and this is favourable for higher dyeability. On the other hand, Simultaneous Oxidation Method takes the shortest treatment time. This is an important advantage of the method.
3.2. Effect of Treatment Temperature
The effect of treatment temperature on the dyeability for each the dyeing method with O2 introduction except for the Sequential Method was investigated in order to improve the dyeability further. In the Post-Oxidation Method, the temperature of the first process and second process was independently controlled. When the first process temperature is controlled from 20˚C to 60˚C, the second process one was fixed at 30˚C. When the second one is controlled from 20˚C to 60˚C, the first one was fixed at 30˚C.
In Redissolution Method, the obtained colour of hair becomes deeper with increasing dyeing temperature (T) and its yellowness and b* are the highest at 40˚C as shown in Figure 5. The L* decreases and a* increases with increasing T. Figure 6 (a) shows the K/S spectra for the hair dyed by Redissolution Method at 20˚C - 60˚C. The strength of the signal at 400 nm increases with the temperature and reaches maximum at 50˚C. It decreases at 60˚C, whereas the K/S values at 440 nm and over slightly increase. The T affects on the dye diffusion may contribute largely to the dyeability with catechinone.
Figure 5 indicates that L* for the hair dyed by Simultaneous Oxidation Method with O2 introduction decreases with T. Its’ a* and b* increase with T up to 40˚C and decrease over 40˚C. The K/S values at 400 nm and over 520 nm increase with T as shown in Figure 6(b). The values at 440 - 500 nm decrease with T over 40˚C. On the one hand, the dye formation is inhibited over 40˚C because tyrosinase is deactivated by heating [9] . On the other hand, the chemical oxidation of Cat with O2 is promoted in some degree at higher T [8] . The dye diffusion may also be accelerated by heating. These mechanisms may cause the dyeing behaviour in Simultaneous Oxidation Method.
![]()
![]()
![]() (a) (b) (c)
(a) (b) (c)
Figure 5. Dyeing temperature (T) dependence of the L* (a), a* (b) and b* (c) for hair dyed by redissolution method with catechinone (
) or simultaneous method with cat ( ) in the presence of O2.
![]()
![]() (a) (b)
(a) (b)
Figure 6. K/S spectra for the hair undyed (---) and dyed by redissolution Method with catechinone (a) or simultaneous method with Cat (b) at 20 (―), 30 (―), 40 (―), 50 (―) or 60˚C (―) in the presence of O2.
In Post-Oxidation Method, the second oxidising process is affected by heating as shown in Figure 7. The L*, a*and b* are almost constant at the different temperatures (T1) of the first process. In contrast, L* increases slightly with increase in the temperature (T2) of the second process over 40˚C, and a* and b* decrease with T2 over 40˚C. Its dyeability is invariable by heating in the first step, whereas the dyeability decreases with an increase in T2. The results show that higher temperature in the first process does not promote the absorption and adsorption of Cat to hair. The drawdown of the dyeability by increasing T2 over 40˚C is mainly due to the deactivation of tyrosinase. The K/S spectra for the hair dyed by Post-Oxidation Method are almost same at all the T1 and show very slightly change with the increase in T2.
The deepest colour of the hair is obtained by Redissolution Method or Simultaneous Oxidation Method at 60˚C. The hair dyed by Simultaneous Oxidation Method at 40˚C shows the most vivid yellow. The dyeability Post-Oxidation Method at lower temperature (20˚C and 30˚C) is comparatively high.
3.3. Proposed Dyeing Mechanism
The mechanisms of hair dyeing by catechinone are complicated. The interaction between the dyestuffs and dye-sites in hair can be van der Waals force, hydrogen bonding and covalent bonding.
In the Redissolution Method and Sequential Method, it is thought that the dyeing process proceeds via 1) dyestuff approaching surface on hair, 2) penetrating and diffusing into hair, 3) being adsorbed onto hair, 4) being fixed at dye-sites in hair.
The Cat reacts with tyrosinase at the active centre in the enzyme and its molecular weight is ca. 130,000, which is too high to penetrate into the hair matrix. Therefore, Cat reacts with tyrosinase and is oxidised in solution, and then dye molecules penetrate into hair and are adsorbed in Simultaneous Oxidation Method. Understandably, Cat penetrates into hair in parallel with the penetration of catechinone. The enzymatic reaction of Cat occurs close to the hair surface in Post-Oxidation Method. The dyeability of Post-Oxidation Method is slightly lower than that of Simultaneous Oxidation Method, because only the Cat molecules, which exist in the vicinity of the hair surface, react with tyrosinase. Other Cat molecules should be supplied from deeper part of the hair. The diffusion rate of Cat molecules in the solution is higher than that in hair.
4. Conclusion
The dyeability of the Simultaneous Oxidation and Post-Oxidation Dyeing Method is higher than that of Redissolution and Sequential Dyeing Method for hair by using (+)-catechin. The higher temperature (40˚C - 60˚C) is favourable for the Simultaneous Oxidation and Redissolution Dyeing Method. The Post-Oxidation Dyeing Methodmade at lower temperature (20˚C - 30˚C) gives higher dyeability. The deactivation of tyrosinase by heating over 50˚C results in decreasing the dyeability for the Simultaneous Oxidation and Post-Oxidation Dyeing Method.
![]()
![]()
![]() (a) (b) (c)
(a) (b) (c)
Figure 7. Adsorption temperature (T1, ) and oxidation temperature (T2, ) dependence of the L* (a), a* (b) and b* (c) for hair dyed by Post-Oxidation Method with Cat in the presence of O2.
Acknowledgements
This study was financially supported partly by the Japan Society for the Promotion of Science as Grant-in-Aid for Young Scientists (B) (No. 15K16185), the Promotion and Mutual Aid Corporation for Private Schools of Japan as Young Researchers Incentives of the Science Research Promotion Fund and The Cosmetology Research Foundation.
NOTES
![]()
*Corresponding author.