Received 18 January 2016; accepted 19 February 2016; published 24 February 2016

1. Introduction
In Kyrgyzstan, the potato (Solanum tuberosum) is a staple product for the population after grains. Many areas in the country have favorable soil and climatic conditions for growing potatoes. The main acreage is located in the Issyk-Kul region, in the mountainous areas of the Fergana Valley, and in the Kemin district of Chui valley. Early varieties are grown in warm areas and conventional varieties in the foothills.
According to the Kyrgyz Republic Plant Protection and Chemicalization Department’s reports (2011, 2012) 25% - 30% of the potato crop is exposed to various rotting during storage, of which the main damage is caused by soft rot.
The E. carotovora ssp. carotovora (Ecc) bacterium is one of the most important factors which cause soft rot of stem and tubers before and after harvest, and greatly reduce yields. The bacteria mainly attack the fleshy storage organs of their hosts (tubers, corms, bulbs, and rhizomes), but they also affect succulent buds, stems, and petiole tissues.
This pathogen has a host range limited almost exclusively to potatoes in temperate regions [1] . E. carotovora ssp. carotovora (Ecc) can be found on plant surfaces and in soil, where it may enter the plant via wound sites or through natural openings on the plant surface, e.g., lenticels. In the vascular tissue and intercellular spaces of suberized tissues, a pathogen remains until environmental conditions, including free water, oxygen availability, and temperature, become suitable for disease development [2] [3] . The main weapon in the soft rot erwinia arsenal is the co-ordinated production of high levels of multiple exoenzymes, including pectinases, cellulases, and proteases, which break down a plant’s cell walls and release nutrients for bacterial growth [4] -[7] . Pectinases are the main exoenzymes involved in disease development. These exoenzymes break down and utilize pectins in the middle lamella and plant cell walls, causing tissue collapse, cell damage, and cell leakage [4] [5] [8] .
Control of bacterial soft rot of vegetables is traditionally based on phytosanitary and cultural practices. Use of chemicals is generally not recommended to control soft rot because of the high risk of the residual effect of toxic chemicals that might be hazardous to consumers’ health [9] .
The importance of environment-friendly plant protection methods is greatly emphasized in sustainable agriculture. The recent increase in publication on bacterial endophytes reflects an interest in their potential benefits as biocontrol agents in agriculture [10] . So the development of environment friendly control measures against soft-rot causing bacteria may minimize loss in storage and improve the quality of potatoes. Biological control is a potential method to control soft rot disease [11] . Biological-control treatments consisting of living microorganisms or abiotic products can provide disease protection, essentially through one or more of the following: 1) production of antibiotics or other molecules that are deleterious to the pathogen’s development, 2) competition with the pathogen for nutrients and space, or 3) induced plant resistance. This method has a more specific effect on the pathogen and has a limited impact on the environment [12] .
The strategy for biological control of plant diseases involves the use of antagonistic microorganisms before or after the infection takes place. Commercial biological control agents are available for the seed treatments and soil amendments to protect the plants against soil-borne pathogens. The potentiality of biological control of bacterial soft rot with antagonistic bacteria or with growth promoting rhizobacteria, fluorescent pseudomonads, and endophytic bacteria in many crops has already been proven [13] [14] . The bioagent B. subtilis was the most effective one in reducing the soft rot decaying stored potato tubers [15] .
In Kyrgyzstan, an environmentally friendly biological control measure to prevent potato damage has still not been developed. For the development of protective measures that could reduce the loss of roots during storage it is necessary to identify pathogens and to study their biology and etiology. The aim of this research was the isolation of soft rot pathogens from different varieties of potatoes during storage, their diagnosis and identification, and evaluation of the antibacterial activity of antagonistic microorganisms in in vitro and in vivo tests against the Erwinia carotovora bacterium for biological control of soft rot.
2. Material and Methods
2.1. Potato Tuber as a Source of Pathogens
The potato tubers of Picasso, Sante, and Nevskiy varieties were used for direct isolation of E. carotovora ssp. carotovora (Ecc) (Table 1). Infected samples of potatoes showing the characteristic symptoms of soft rot were taken from storage area. Samples were brought into the laboratory in polythene bags.
![]()
Table 1. Potato varieties used for research.
2.2. Isolation and Purification of the Pathogen Organism
Infected samples were cut into small pieces of 2 - 3 cm length and their surfaces sterilized with 1% HgCl2 for 2 - 3 minutes with three successive washings in distilled water. The sterilized pieces were placed on Petri plates containing a Potato Dextrose Agar (PDA) medium (20 ml/dish). The plates were incubated at 28˚C for five to seven days.
2.3. Identification of the Pathogen Organism
Morphological tests on the shape and motility of cell, and physiological and biochemical tests were conducted. Cavity formation on a crystal violet pectate (CVP) medium and fluorescence on King’s B medium were performed, as described earlier [16] [17] . The tests used in this study were Gram staining [18] , growth in 1% - 5% NaCL, and at 37˚C performed as described in Bergey’s Manual of Systematic Bacteriology [19] . Catalase and Nitrate test [20] , Starch hydrolysis test [21] , V.P test, Methyl read reaction and H2S production [22] and acid production from carbohydrates utilized as a source of carbon glucose, maltose, sucrose, lactose, inositol and α-methyl glycoside, amylolysis, utilization of citrate. The ability to grow at 27˚C, 33.5˚C, and 37˚C was also assessed.
2.4. PCR Analyses
DNA isolation, restriction, ligation, and agarose gel electrophoresis was carried out according to standard protocols. Amplification of Erwinia carotovora ssp. carotovora was performed with primers MseF GACGATGAG TCCTGAG and MseR TACTCAGGACTCAT.
2.5. Characteristics of Biocontrol Agents
The antagonistic microorganisms used in this study―Streptomyces species, Bacillus species, and Trichoderma lignorium―were obtained from the laboratory collection of the Phytopathology Laboratory (Plant Protection Department, Faculty of Agriculture, Kyrgyz-Turkish Manas University, Kyrgyzstan). Streptomyces diastatochromogenes strain sk-6 was isolated from the rhizosphere of wild plants in an elevated mountain ecosystem (3400 m above sea level) and Streptomyces graminearus strain sk-2 was isolated from the rhizosphere of garlic in agrobiozenose. The 16S rRNA genes of these strains were PCR amplified with 27f and 1522r primers. Bacillus cereus and Bac. polymyxa strains were isolated from soils contaminated with high concentrations of heavy metals and Trichoderma lignorium was isolated from soil where red beets were grown. They have been selected as active antagonistic organisms after successive screenings against gram positive and gram negative bacteria, also pathogen fungi.
2.6. Test on the Pathogenicity of E. carotovora ssp. carotovora (Ecc) Strains to Host Plants by Artificial Inoculation
To determine the pathogenicity of E. carotovora ssp. carotovora (Ecc) strains, healthy potato tubers were used. They were washed first in piped and sterile water, then were sterilized in 96% ethanol and dried on the surface of the filter paper. Of these, discs 3 - 5 mm thick and 1 cm in diameter were prepared, placed on the surface of a 1.5% agar medium in a Petri dish, and kept in a humidified chamber. Discs were coated with Ecc culture (107 - 108 cells/ml) and incubated overnight at 28˚C. In addition to testing the pathogenicity of the bacteria, healthy carrots discs 5 cm in height were used in the experiment. For this purpose, they were sterilized in 96% ethanol and dried on the surface of sterile filter paper. In each disc was made a notch of 3 cm depth and 5 cm in diameter. Ecc suspension was added at three different concentrations inside these dimples. Inoculated carrot disks were incubated at 24˚C - 25˚C. In the control variant, sterile water was used instead of the bacterial suspension. The development of the disease was checked at periods of 2.4, 7, and 14 days.
2.7. In Vitro Antimicrobial Activity of Biocontrol Microorganisms against E. carotovora ssp. carotovora (Ecc) Strains
The antagonistic microorganisms and soft rot isolates were grown in nutrient glucose agar (NGA) slants for 48 hours at 28˚C, then suspended in 3 - 5 ml of sterile distilled water, and turbidity was adjusted metrically to approximately (107 CFU/cm3) for all soft rot isolates.
A loopful of fresh culture of each antagonist was streaked as a single line on the middle of the plates, and then incubated at 28˚C for 48 hours. The tested soft rot isolates were streaked in lines perpendicular and down to the line of antagonist. Plates were incubated at 28˚C for 48 hours, and bacterial growth of Ecc isolates or inhibition zones were measured as the distances between the edge of antagonistic bacterial growth and the edge of Ecc tested isolates using the method described by [23] . Data were recorded after 48 hours.
2.8. Evaluation of the Inhibitory Effect of Antagonistic Bacteria under Storage Conditions
To evaluate the effectiveness of the selected antagonistic bacteria in reducing soft rot infection in storage potatoes, 700 g of fresh tubers of each of the three potato varieties Nevskiy, Pikasso, and Sante were dipped in suspensions of the antagonistic bacterium Streptomyces diastatochromogenes sk-6 (104 - 108 spore/ml) for 30 min and air-dried separately. The treated potato tubers were inoculated with soft rot bacteria Ecc isolates by spraying them with inoculum suspensions (104 - 108 spore/ml). Inoculated potato tubers bulbs were air-dried and stored separately at room temperature. Data on soft rot incidence were recorded after 1, 2, 3, 4, and 7 weeks of inoculation. Number and weight of soft-rot infected tubers were recorded and expressed in percentages using the following formula described by Abd-El-Khair and Karima [24] :
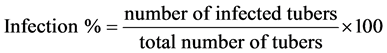 .
.
Percentage of disease reduction (PDR) was calculated according to the following formula (Hajhamed et al. 2007):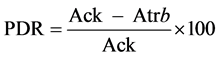 , where Ack and Atr represent the severity of the disease in control and treated
, where Ack and Atr represent the severity of the disease in control and treated
samples, respectively [25] .
Statistical analysis: Data were statistically analyzed using a computer program, Statistical Analysis System (SAS) [26] .
3. Results and Discussion
3.1. Cultures of Soft Rot, Erwinia carotovora ssp. carotovora (Ecc) Isolates
Ten isolates of Erwinia carotovora ssp. carotovora (Ecc) were isolated from infected potato tubers of Picasso, Sante, and Nevskiy varieties collected from different regions in Kyrgyzstan. Isolates were identified as Erwinia carotovora ssp. carotovora (Ecc) by standard bacteriological techniques and pathogenicity tests on tubers, also by PCR analyses.
The isolated bacteria were identified as Erwinia carotovora ssp. carotovora according to its morphological and biochemical character. The bacterium was rod shaped with rounded ends, convex, and creamy white colonies of cells appeared both singly and in pairs. The isolated bacteria were Gram negative. No results were obtained with Starch hydrolysis, Nitrate reduction, and V.P tests. Positive results were obtained with methyl red reaction, indole formation, and bacteria were grown under anaerobic conditions. The production of acid from fructose, galactose, and glucose yielded positive results but negative ones for arabinose, lactose, and maltose. Intensive growth occurred at 28˚C and 30˚C over 24 hours, while at 36˚C after 72 hours very weak growth was observed. Decomposition of gelatin was observed in the form of the recess in the thickness of the nutrient medium for seven to 10 days at room temperature.
3.2. The Pathogenicity of E. carotovora ssp. carotovora to Host Plants by Artificial Inoculation
After four days, all infected potato tuber samples began to show the symptoms of the disease. On day 14 all contaminated samples had been covered by visible rings with black lines from the middle toward the edges of the discs. In the variety of Picasso, the symptoms occupied 50% - 80% of the tubers in 12 days (Table 2). This variety is grown in all regions of Kyrgyzstan and our results have shown that this pathogen is widespread in these areas. In control samples of the disease, symptoms were not observed. As results have shown, the potato tubers of the Picasso variety were more damaged by an artificial infection of E. carotovora ssp. carotovora in the short-term than other varieties (Figure 1). Therefore, this variety is more sensitive and susceptible to the disease. In addition to potato tuber, this pathogen also infected the carrots tubers (Figure 2). As a result of artificial inoculation five isolates were chosen, three isolates (EcPo1, EcPo2, and Eco3) were highly pathogenic, while two isolates (Eco4 and Eco5) were weakly pathogenic.
3.3. Sensitivity of Erwinia carotovora ssp. carotovora Isolates to Antagonistic Microorganisms (in Vitro)
Data presented in Figure 3 show that the used antagonistic microorganisms had different inhibitory effects against isolates Erwinia carotovora ssp. carotovora (Ecc). The antagonistic bacteria Streptomyces diastatochromogenes strain sk-6, and Streptomyces graminearus strain sk-2 had a highly significant effect on soft rot
![]()
Table 2. Pathogenicity of E. carotovora ssp. carotovora isolates to host plants by artificial inoculation.
“−” = invisible symptoms, “+” = the symptoms appeared clear, “+−” = symptoms slightly noticeable, “++” = 20% symptoms, “+++” = symptoms of 50%, “++++” = symptoms of 80%.
![]()
![]() (a) (b)
(a) (b)
Figure 1. (a) Four days after inoculation with sterile water (control); (b) Four days after inoculation with E. carotovora ssp. carotovora (Picasso varieties).
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) The carrot tubers at the beginning of inoculation with different dosages of E. carotovora ssp. carotovora; (b) 14 days after inoculation.
![]()
Figure 3. Comparative efficiency of biocontrol agents against Erwinia carotovora ssp. carotovora isolates after five days: 8.2 mm-Streptomyces diastatochromogenes strain sk-6; 3.2 mm-Streptomyces graminearus strain sk-2; 2.0 mm-Bacillus cereus strain 4b; 4.3 mm-Trihoderma lignorium.
bacteria isolates (Ecc) than other tested antagonistic organisms. The diameter of the inhibition zones around the antagonistic Streptomyces diastatochromogenes sk-6 colonies and Erwinia strains EcPo1, EcPo2 and Eco3 was 8.0 - 8.2 ± 0.97 mm in triplicates in vitro experiments at five days. The diameter of the inhibition zones around the antagonistic Streptomyces graminearus sk-2 colonies and Erwinia carotovora strains EcPo1, EcPo2, and Eco3 was 3.0 - 3.2 ± 0.89 mm in triplicates at five days (Figure 4). The diameter of the inhibition zones around the antagonistic Trihoderma lignorium colonies and Erwinia carotovora strains EcPo2 and Eco3 was 4.2 - 4.3 ± 0.73 mm in triplicates at five days. The antagonistic bacteria Bacillus cereus strain 4b had fewer antagonistic activities towards Erwinia carotovora strains EcPo2 and Eco3 at five day, while the antagonistic Bacillus polymyxa strain P100 had no effect on soft rot bacteria isolates (Figure 5).
3.4. The Effect of Antagonistic Streptomyces diastatochromogenes sk-6 on Storage Potato Tubers
Streptomyces diastatochromogenes sk-6 was selected for the control assay of storage potatoes against the most common soft rot bacterial strain in Kyrgyzstan, Erwinia carotovora ssp. carotovora EcPo2.
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) Antagonistic activity of Streptomyces diastatochromogenes sk-6 showing inhibition zones against potato soft rot bacterial strain EcPo2; and (b) Streptomyces graminearus strain sk-2 showing inhibition zones against potato soft rot bacterial strain EcPo2.
![]()
![]() (a) (b)
(a) (b)
Figure 5. (a) No significant antagonistic activity of Bacillus cereus strain 4b against potato soft rot bacterial strain EcPo2; and (b) Trihoderma lignorium showing inhibition zones and hyper parasitic effect against potato soft rot bacterial strain EcPo2.
No infection was observed on the fourth week after the inoculation of soft rot bacterium EcPo2 and treatment with the antagonistic bacterium Streptomyces diastatochromogenes sk-6, both using doses of 106 and 108 spore/ml in the Sante variety. The tuber disks have formed buds and the developing mycelia of the antagonist actynomycete have completely covered the surface of the tubers, whereas when using a lower dose of 104 cells/ml, the symptoms of soft rot were observed in 3.0% of tubers in storage conditions.
In the Nevskiy variety, using a dose of 106 and 108 spore/ml after four weeks resulted in no progression of the infection, but with a dose of 104 spore/ml the infection had started to develop, appearing at the edge of the disc as black border stripes.
In the variety of Picasso the signs of infection started to show after four weeks, even with a dose of 108 spore/ml of the antagonist, and the infection symptoms were more pronounced and were observed in 4.5% - 4.7% of this variety’s tubers at a dose of 104 cells/ml of antagonist. The control group or untreated potatoes of all varieties incurred 100% damage within four weeks of the experiment beginning (Figure 6).
![]()
![]() (a) (b)
(a) (b)![]() (c)
(c)
Figure 6. Effect of antagonistic bacteria Streptomyces diastatochromogenes sk-6 on soft-rot incidence of potatoes in storage, as observed after four weeks at different antagonist doses: 1. 108 cells/ml; 2. 106 cells/ml; 3. 104 cells/ml.
Figure 7 shows that the disease symptoms were developed in 5.0% - 5.2% of the Sante and Nevskiy varieties’ tubers after five weeks when using 108 cells/ml and 106 cells/ml doses of the antagonist bacteria, whereas when using a lower dose of 104 cells/ml the symptoms of soft rot were observed in 7.5% and 8.0% of tubers in storage conditions. The highest infection rate (35% - 40%) was observed in the Picasso variety at a dose of 104 cells/ml of the antagonist. This indicates the high sensitivity of this variety to soft rot in storage conditions, which is shown more clearly when using a low dose of a biological agent. Therefore, as the results have shown that the development of infection depends on the doses of the antagonist microorganism used, the higher the density of developing mycelia of the antagonist, the weaker the development of infection as a result of soft rot (Figure 8(a), Figure 8(b)).
The pretreatment of potato tubers with antagonistic bacteria successfully prevented the initial infection of the multiplication of soft rot bacteria and reduced soft rot disease of potatoes in storage.
These results justify the dose of 106 cells/ml of the bacteria Streptomyces diastatochromogenes sk-6 for use in powdering the infected or non-infected potato tubers to suppress the development of soft rot during storage, because the data have shown that inhibition of the development of this disease under storage conditions directly depends on the concentration or density of the biological agent Streptomyces diastatochromogenes sk-6. Streptomyces play a key role due to their ability to produce numerous different polyketides. Polyketides have attracted great attention since these compounds have been widely applied as antibacterial drugs in medicine. Recently, some polyketides such as validamycin, venturicidin, trichodermin, and nikkomycin have been shown to be active against plant pathogen fungi and have been widely applied as important biocontrol products [27] -[29] .
![]()
![]() (a) (b)
(a) (b)![]() (c)
(c)
Figure 7. Effect of antagonistic bacteria Streptomyces diastatochromogenes sk-6 on soft rot incidence of potatoes in storage as observed after five weeks at different antagonist doses: 1. 108 cells/ml; 2. 106 cells/ml; 3. 104 cells/ml.
![]()
![]() (a) (b)
(a) (b)
Figure 8. (a) Tuber infected with E. carotovora (108 spore/ml) + Streptomyces diastatochromogenes sk-6 (104 cells/ml); (b) Tuber infected with E. carotovora (108 spore/ml) + Streptomyces diastatochromogenes sk-6 (106 cells/ml) after five weeks.
Moreover, the genome analyses of Streptomyces subspecies demonstrated that the isolates possess many PKS genes with unknown functions [30] [31] .
Thus, Streptomyces seem to have the possibility to produce hitherto unidentified polyketides against plant pathogens [32] . Oligomycins are macrolides created by Streptomyces diastatochromogenes that are found in four isomers, namely A, B, C, and D, and are highly specific for the disruption of mitochondrial metabolism. Oligomycin A (Oli-A) could induce apoptosis in a variety of cell types, make cells more susceptible to cell death, and also lead to a switch in the death mode from apoptosis to necrosis. Oli-A exhibits a broad biological profile including antifungal, antitumor, and nematocidal activities [33] . Our results offer the first scientific proof that this antagonist organism shows a strong inhibitory effect on phytopathogenic bacteria producing disease in plants. Therefore, development of new plant protection means that Oli-A has more prospects.
4. Conclusions
For the first time in Kyrgyzstan we have identified the Erwinia carotovora subspecies isolates from different potato varieties by using the biochemical tests, pathogenicity tests, and PCR analysis.
The Streptomyces diastatochromogenes sk-6 was a strong antagonist of soft rot bacteria E. carotovora ssp. carotovora in 106 spore/ml dose. This was confirmed through the in vitro and storage experiments. The ability of this isolate to suppress the growth of phytopathogenic bacteria E. carotovora ssp. carotovora makes it a potential biocontrol agent for reducing the soft rot infection of potato tubers in the storage period. Streptomyces diastatochromogenes sk-6 as a biological disinfectant could destroy surface and internal infection, protect the tubers from the growth of phytopathogenic bacteria in the early period of their reproduction, and improve the overwintering of winter crops.