Stationary Flow of Blood in a Rigid Vessel in the Presence of an External Magnetic Field: Considerations about the Forces and Wall Shear Stresses ()
Received 27 November 2015; accepted 2 February 2016; published 5 February 2016

1. Introduction
The magnetohydrodynamics laws govern the motion of a conducting fluid, such as blood, in an externally applied static magnetic field B0. When an artery is exposed to a magnetic field, the blood charged particles are deviated by the Lorentz force thus inducing electrical currents and voltages along the vessel walls and in the neighboring tissues. Such a situation may occur in several biomedical applications: magnetic resonance imaging (MRI) [1] -[5] , magnetic drug transport and targeting [6] -[9] , tissue engineering [10] -[13] …
An optimal modelisation of the magnetohydrodynamic flow of blood should include the pulsatility of flow, the deformability and conductivity of the vessel wall, together with the induced electrostatic and electromagnetic fields. This leads to a complex mathematical problem and analytical solutions may be found only under restrictive hypotheses.
The analysis presented in this paper is based on the exact solution given by Gold [14] for the stationary blood flow in a rigid vessel with insulating wall in the presence of an external static magnetic field. This analysis completes a previous one [15] . Since the magnetic field breaks the flow axisymmetry, it is interesting to study in details the velocities, pressure, forces, induced fields and currents in a vessel cross section. This is the aim of this paper. Such integrations over the cross-section of the vessel would also be necessary in order to explore the feasibility of deriving 1-D equations [16] for this type of flow.
2. Position of the Problem
As explained by Gold [14] and by Abi-Abdallah et al. [15] , the Navier-Stokes equations including the Lorentz force j^B (1), coupled with the induction Equation (2) govern the flow of a conducting, incompressible, Newtonian fluid in an externally applied static magnetic field B0.
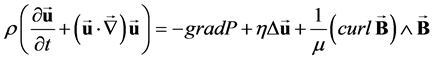 (1)
(1)
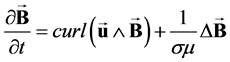 (2)
(2)
where u and P are the fluid velocity and pressure, t is the time, j = (curlB)/μ is the electric current density; μ is the magnetic permeability; ρ, η and σ are the fluid density, viscosity and conductivity. The magnetization force that could be induced in the blood by magnetic field gradients is neglected in this study since B0 is uniform and the induced magnetic field BI(r, θ) is very small [17] .
Using the identity:
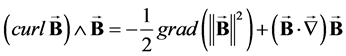 (3)
(3)
it is possible to write (1) in the form:
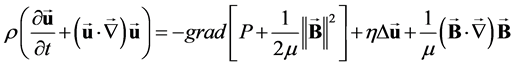 (4)
(4)
thus introducing the magnetic pressure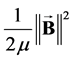 .
.
3. The Case of a Unidirectional Steady Blood Flow in a Rigid Circular Vessel with Insulating Walls
Gold [14] considered the case of a unidirectional steady blood flow in a rigid circular vessel with insulating walls (Figure 1, from Abi-Abdallah et al. [15] ). The velocity and magnetic field are defined in the cylindrical frame (er, eθ, ez) as:
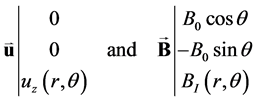 (5)
(5)
![]()
Figure 1. Inductions in the vessel. The induced currents circulate in a closed loop in the transverse plane. The induced field is parallel to the Oz axis with opposite directions on each side of Oy.
The induced magnetic field is parallel to the flow and guarantees div B = 0. The continuity equation div u = 0 is also satisfied. The charge separation is supposed to arise in a plane which is perpendicular to the velocity, thus inducing an electric field that is located in that same plane. The induced current in the tube center is oriented upward as shown, and the return currents adjacent to the walls are oriented downward. In the tube center, j and B are directed so that the ponderomotive force is opposed to the fluid flow. Near the walls, however, the return currents are in the opposite direction, thus the fluid flow is enhanced by the ponderomotive force that is generated by the return currents. The currents cannot escape the vessel because the wall is insulating. The induced magnetic field results from these induced currents flowing in closed loops (Biot and Savart law).
The projection of (1) in the cylindrical frame is thus:
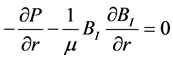 (6a)
(6a)
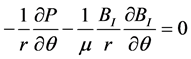 (6b)
(6b)
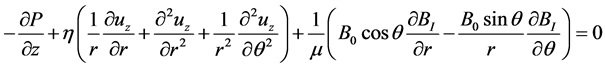 (6c)
(6c)
The projections on er and eθ (6a) and (6b) may also be written:
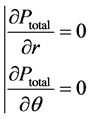 with
with 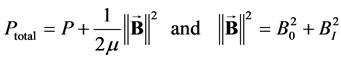 (7)
(7)
It appears from (7) that Ptotal is uniform in a transverse section of the vessel (whereas P is not) and that
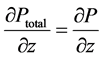 (8)
(8)
Of course, the projection of (4) also leads to (6)-(8).
The induction Equation (2) has only one interesting projection: the longitudinal one (along ez):
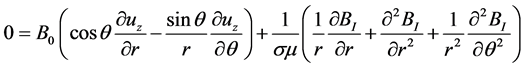 (9)
(9)
4. Integration of the Equations over a Transverse Section of the Vessel
We are now interested in integrating the longitudinal projection in (6) and (9) over a transverse section of the vessel.
Using the identity (A1) established in Appendix A, it is possible to show that:
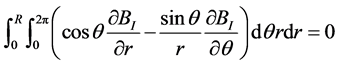 (10)
(10)
because,
![]() (11)
(11)
and BI(R, θ) = 0 for a non-conducting wall.
Equation (10) means that the integration over a cross section of the vessel of the longitudinal projection of the Lorentz force is zero:
![]() (12)
(12)
This seems related to the existence of current return paths (Figure 1). The contributions of these currents compensate each other over the section, and the total contribution is null. In the case of neglected induced fields or electrically conducting channels, the recirculating loops do not exist and this result is no longer valid.
Using the same identity (A1; Appendix A), it is also possible to show that:
![]() (13)
(13)
because,
![]() (14)
(14)
and uz(R, θ) = 0 (no-slip condition at the rigid wall).
Integration of (6c) and (9) over a cross section of the vessel thus yields:
![]() (15)
(15)
This shows that the integral of Δuz over the section is independent of B0, whereas the mean velocity (integral of uz over the section) depends on B0 (see Graphs no. 5 and no. 18 in Abi-Abdallah et al. [15] ).
Using the identity (A2) of the Appendix A, (15) become:
![]() (16)
(16)
![]() (17)
(17)
Considering that:![]() , we have:
, we have:
![]() (18)
(18)
and (17) means that:
![]() (19)
(19)
This result can be checked in the Graphs no. 10 of Abi-Abdallah et al. [15] and is coherent with the current compensation that is illustrated in Figure 1 of this paper.
We can also notice that, in a hypothetic case where the gradient of velocity at the wall would not depend on θ, (16) would reduce to a classical balance of forces on a small volume element of unit length:
![]() (20)
(20)
Equation (16) thus demonstrates that the statements of the viscous stresses have to be reconsidered in the case of MHD flow, because the velocity uz depends on r and on θ. This can be understood looking at the Graphs no. 3 in Abi-Abdallah et al. [15] . This Figure presents the iso-velocity lines in a transversal plane of the vessel. One can observe that the presence of a static magnetic field reduces the blood flow and flattens the velocity profile. This appears more clearly for B0 > 20T. The profile is stretched parallel to the direction of B0 (θ = 0). The iso-velocity lines are more tightened in the position θ = 0 than in the position θ = π/2, indicating that the velocity gradient ∂uz/∂r is higher for θ = 0 than for θ = π/2. This gradient is also higher when B0 increases (in the position θ = 0, the lines are more and more tightened for higher values of B0). The same conclusions can be drawn when looking at the Graphs no. 4 in Abi-Abdallah et al. [15] . In this figure, it appears more clearly that the velocity profile becomes more and more flat when B0 increases, with thin boundary layers (called “Hartmann boundary layers”) at the walls, where viscous drag drives the flow to zero. It is in the Hartmann layers that the electric currents, induced at the core flow in the y-direction, return and close the current loop. In an electrically insulated channel, the modification of the parabolic laminar velocity profile increases shear friction at the walls, which in turn increases (∂P/∂z). This is what (16) shows.
It could be possible to confirm these qualitative observations by calculating ∂uz/∂r and ∂uz/∂θ from Equation (14) of Abi-Abdallah et al. [15] . Evaluating these quantities at the wall should allow to see whether the stresses reach some levels that could be at risk: 1) for the vessel wall (plaque rupture in case of atherosclerotic lesion [18] , severity of some aneurysms [19] , ...), 2) for the mechanotransduction in the endothelial cells, 3) for other cells attachment and/or transmigration (white blood cells, tumor cells, cells seeded in vascular substitutes [20] , ...). Estimation of shear stresses at the wall may also have important implications in magnetic nanoparticle delivery and drug targeting [6] [21] .
5. Conclusion
In this paper, we have considered the steady unidirectional blood flow in a circular rigid vessel with insulating walls, in the presence of an exterior magnetic field. The exact solution of Gold [14] (with the induced fields not neglected) has been explored. It has been shown that the integration over a cross section of the vessel of the longitudinal projection of the Lorentz force is zero, and that this result is related to the existence of current return paths, whose contributions compensate each other over the section. It has been also demonstrated that the classical definition of the shear stresses cannot apply in this situation of magnetohydrodynamic flow, because, due to the existence of the Lorentz force, the axisymmetry is broken.
Acknowledgements
The authors would like to thank Professor Yuri Molodtsof, from University of Technology of Compiègne (UTC), for his helpful comments on this work.
Competing Interests
None.
Ethical Approval
Not required.
Appendix A
For every smooth function f(r, θ), it is possible to establish the following two identities:
Identity (A1): ![]()
Proof:
![]()
Identity (A2): ![]()
Proof:
![]()