Received 6 November 2015; accepted 10 January 2016; published 13 January 2016

1. Introduction
While 50-60-ies of XX century, the main toxic air pollution, were made of industrial and domestic companies, the level of emissions of toxic components into the environment of internal combustion engines (ICE) of last decade has exceeded many industrial facilities as a fact of rapid growth in the number of cars and trucks (currently in the world there are over 450 million vehicles units).
While improving of motor fuel quality and design of internal combustion engines achieved by countries of the “far” and “near” abroad has significantly improved environmental characteristics of used vehicle park, however, the massive use of catalytic converters of exhaust gases should be considered the most important protection measure ensured environmental compliance of vehicles [1] .
Previously, we have performed a comprehensive study on the development of effective catalysts for deep oxidation of hydrocarbons and carbon monoxide in the exhaust gas carburettor, containing the active composition which is not deficient oxides of copper, chromium, cobalt and manganese on the surface volumetrically structured Al2O3/A1-frame carriers [2] .
It is shown that the highest activity in the oxidative conversion of carbon monoxide exhibit catalytic systems is characterized by a maximum content of secondary alumina coating phase chromate copper (CuCrO4), while as for deep oxidative conversion of hydrocarbon (n-butane) responsible phase chromite cobalt (CoCr2O4). To realize the optimum profile of the macroscopic-ray distribution in the matrix phase, designated carrier has been proposed a method for separate application of opposite binary combinations of active metals, described in detail in [3] .
However, heat treatment of potential catalysts under traditional heat transfer due to the presence of a temperature gradient from the surface of the carrier granules to the center, occurs uneven distribution of active components, and as a consequence, the generated samples exhibit poorly reproducible catalytic activity [4] .
In this regard, it should be noted that a distinct advantage of the method of the microwave heat treatment of materials has spread in recent years in the field of high technologies such as the preparation of heterogeneous catalysts and catalytic transformations of the further stimulated by microwave radiation [5] [6] .
In this paper, there was an attempt to intensify the process of preparation of Cu-Cr-Co/Al2O3/Al-catalysts joint deep oxidation of hydrocarbons and mono-oxide, carbon (for example, the conversion of n-butane and CO) through the formation of catalytically active phase under the action of the microwave field and holding reaction at stimulating effects of microwave radiation.
The task of selecting the component composition of the catalysts for the reaction indicated in a microwave field, thermal treatment was significantly facilitated by the development of the formulation of cooking in a conventional heating [7] .
2. Methods and Apparatus
Microwave brand EM-G5593V (Panasonic) with the volume of the cavity 23 liters. functioning at an operating frequency of 2450 MHz, with a maximum input power of the radiation generator (magnetron) of 800 watts. X-ray diffractometer DRON-3 with a graphite monochromator. Laboratory microwave brand NE-1064F (Panasonic) with the volume of the resonator 14 liters.
3. Experimental
Samples of the catalysts were prepared by impregnation (incipient wetness) absorbing the microwave radiation is γ-A12O3/Al-carrier nitrates of copper, chromium and cobalt-based content of the oxide forms of metals in the active mass (calculated as oxides CuO, Cr2O3 and Co3O4), respectively, 53%, 32% and 15% (wt.), at a total ratio of metal oxide to the weight of active γ-A12O3/Al-carrier of 10%, 15% and 20% (wt.).
Heat treatment of the samples was carried out at the facility, constructed on the basis of microwave brand EM-G5593V (Panasonic) with the volume of the cavity 23 liters. functioning at an operating frequency of 2450 MHz with a maximum input power of the radiation generator (magnetron) of 800 watts. The technical capabilities of the microwave oven allows for heat treatment as a normal sample electrically heated coil, so and programmed to vary the ratio of power of microwave and electric heating. To avoid overheating the sample in the cavity furnace installed capacity of the circulating flow of distilled water. For X-ray diffraction patterns of the powders was applied to glass and instrumentation fixed with varnish, which has its own structural reflections.
X-ray diffraction powder samples were obtained on an automated X-ray diffractometer DRON-3 with a graphite monochromator.
The measurements were carried out on CuKα radiation in step-scan mode with a step 2θ = 0, 1˚. The exposure time per point is 3 seconds. Treatment-togramm diffraction was carried out using software for qualitative and quantitative X-ray analysis [8] .
Temperature-reduced catalysts was investigated by the method described in [9] .
Experiments to assess the penetration depth of microwave radiation into the charge Ni-Co-Cr/Al2O3/Al-cata- lyst and its termotransformatsionnyh properties conducted on an apparatus constructed based on laboratory microwave brand NE-1064F (Panasonic) with the volume of the resonator 14 liters [10] .
Specific surface of the samples were examined for the device “Sorbie-MS” BET.
4. Results and Discussion
Figure 1 illustrates the dynamics of changes in temperature of samples of potential catalysts in the process of heat treatment due to the absorption of microwave energy radiation with a frequency 2450 MHz varying power. For comparison, shows the dynamics of changes of sample temperature γ-A12O3/Al-carrier under microwave irradiation prior to impregnation with a solution of salts of the active metal directly after the hydrothermal treatment step, the curve (2).
It can be seen that regardless of the component composition of the active mass in the first few minutes of exposure wet sample due to the high value of the dielectric loss intensively absorb microwave energy even when the power of the magnetron, to be ~30% of maximum. At the same time, a high rate of heating of samples (about 25 - 30 K/min.) The intense evaporation leads (due to heat) to reduce the temperature of the samples, and for this reason the initial phase of the microwave heating of the dynamics of change in temperature is extreme.
After completion of the drying process, the samples impregnated with the appropriate metal salts, raise the magnetron power of 300 to 600 Wt. It leads to an extreme increase in temperature with highs in the range 450 - 500 K.
The observed changes in the dynamics of the sample temperature in that range is associated with an exothermic reaction thermolysis nitrates of copper, chromium and cobalt in an air atmosphere (at the outlet of the catalyst with the charge capacity observed efflux dyed brown nitrogen oxides). Further increases in radiation power
![]()
Figure 1. Influence of power microwave radiation (1); the dynamics of change in tempera tours samples γ-A12O3/Al carrier in the drying process―(2); Media samples simultaneously impregnated with a solution of nitrate Cu, Cr, and Co―(3); samples prepared by the method of separate application of binary combinations (Cu and Cr) and (Cr and Co) based oxides, the amount of content in the matrix media 20% (wt.)―4.
results in an increase in temperature with entry temperature region start the formation of active metal oxide phase (623 - 653 K) [9] [10] .
Exposure to microwave field patterns γ-A12O3/Al-carrier subjected single-stageimpregnation of a mixture of nitrates of Cu, Cr, and Co (curve 3) results in a less intensive heating the samples when the power of magnetron 600 to 800 Wt. In the case of the samples, obtained by the application of separate components of the active mass (curve 4) observed a higher rate of temperature rise, with comparable values of the magnetron power.
This fact is probably related to an event during heat treatment of the samples the formation of the phase composition of oxide forms of the active metals are characterized by a relatively large amount of dielectric loss.
On radiographs presented in Figure 2 there is qualitative agreement reflexes oxide phases, formed in conditions of traditional heat treatment of the samples (range A) and under microwave irradiation (spectra B and C).
Comparison of the X-ray samples synthesized by a joint (simultaneous spectrum B) and separate (consecutive spectrum C) the introduction of the matrix carrier oxides of active metals allowed to come to the conclusion that the latter are implemented more favorable conditions for the formation of catalytically active phase chromite, cobalt (CoCr2O4) and copper chromate (CuCrO4), as evidenced more intensive reflections in the spectrum of the data phase, evidenced reflexes phase data in the spectrum (C) a relatively large area.
The proximity of the qualitative composition of the oxide forms of metals, are part of an asset-term surface of the Cu-Cr-Co/Al2O3/Al-catalysts also follows from a comparison of the spectra of the temperature-programmed reduction of samples prepared by thermal treatment of electric heating [4] , and under the influence of the microwave field (Figure 3).
![]()
Figure 2. The XRD pattern of samples of Cu-Cr-Co/Al2O3/Al-catalysts obtained by impregnation step Al2O3/Al-Cu nitrate medium, Cr and Co in a conventional heat treatment (A); obtained by heat treatment under conditions of microwave-radia- tion for one-step impregnation of Al2O3/Al-Cu nitrate medium, Cr and Co (B); obtained under step impregnation of the support by binary combinations of nitrates of Cu-Cr and Co-Cr (C).
![]()
Figure 3. The curves of temperature-programmed reduction of samples of Cu-Cr-Co/Al2O3/Al- catalysts obtained by heat treatment electrical heating (a) and in the microwave field (b).
It is seen that the absorption maxima are observed temperature hydrogen reduction of the oxides in the Cr (VI) (403 K) and Cu (II) (523 K) in phase chromate copper (CuCrO4); Cr (III) (593 K) in the phase of copper chromite (CuCr2O4); Co (II) (478 K) in the phase of cobalt chromite (CoCr2O4); Co (II) (678 K) in the oxide-cobalt oxide (Co3O4); Cr (III) (758) in the chromium oxide Cr2O3 for both types of samples are in close agreement.
It should be noted that the completion of the phase formation of chromate copper and cobalt chromium, responsible for the conversion of carbon monoxide and n-butane in a conventional heat treatment the Cu-Cr-Co/ Al2O3/Al-catalysts electric heating occurs at a temperature of 773 - 773 K for 6 - 7 h., while the formation of catalytically active phases CuCrO4 and CoCr2O4 samples during heat treatment in the microwave field occurs within 25 to 30 minutes.
It was also found Figure 1 that the samples of Cu-Cr-Co/Al2O3/Al-catalyst formed under conditions conducive to the formation of a maximum in the matrix of the secondary carrier phase CuCrO4 and CoCr2O4 characterized by a sufficiently high intensity of heat due to the absorption of microwave energy field, increases with increasing content in samples of the active metal oxides. The temperature in the reaction device by transforming the absorbed electromagnetic energy reaches (depending on the amount of supported oxides) values of the order of 600 - 800 K, and stabilized by establishing heat balance with the environment.
Thus, the results suggest that the formation of the Cu-Cr-Co/Al2O3/Al-catalysts joint deep oxidation of hydrocarbons and CO matched component composition using a heat treatment in a microwave field is acceptable for practical implementation, and from the point of view saving time and energy cost compares favorably with the traditional processes of heat.
It is known that in order to achieve the necessary temperature to activate the reaction system under microwave irradiation requires a high level of absorption of the catalyst the charge energy microwave field and its transformation into heat. Also, a significant factor affecting the uniformity of the heating of the catalyst bed is the depth of penetration of the microwave radiation.
Figure 4 is a bar graph illustrating the thermal transformation properties of samples Cu-Cr-Co/Al2O3/Al- catalyst and penetration depth of the volume of the microwave radiation. For comparison, the characteristics of Al2O3/Al-carrier before applying the active composition (A)
It is seen that the initial capacity loss of radiation for samples prepared by single-step impregnation of Al2O3/ Al-medium slightly exceeds the weight loss of the carrier, in while samples prepared by separate impregnation exceed said samples by this parameter.
![]()
Figure 4. Thermo transformation properties and the depth of penetration of microwave radiation: in a lot of Al2O3/Al-carrier before applying the active ingredients (A); catalyst prepared under microwave irradiation method of the single-step impregnation of the support with nitrates Cu, Cr and Co (B); catalyst prepared by the method of time-limiting (step) impregnation with a fractional separation of the components (B). Terms: PBX magnetron = 800 wt., the exposure time of 2.5 minutes.
The level of the microwave radiation power loss (ΔР) in the case of samples prepared by the method of separate application of active ingredients is sufficient, in accordance with the expression given in [11] [12] :
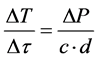 (1)
(1)
where: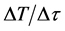 ―rate of temperature rise (K/sec.), the power loss is proportional to the radiation of the magnetron (ΔР); c―average heat capacity of the sample (kcal/deg・mol); d―density (g/sm3), for a few minutes to ensure the temperature of the “ignition” of the catalyst―473K and above.
―rate of temperature rise (K/sec.), the power loss is proportional to the radiation of the magnetron (ΔР); c―average heat capacity of the sample (kcal/deg・mol); d―density (g/sm3), for a few minutes to ensure the temperature of the “ignition” of the catalyst―473K and above.
In the same sufficiently to ensure a uniform (no gradient), temperature distribution in the catalyst bed is the penetration depth of microwave radiation δ, ~70 sm.
As seen from the data presented in Table 1, samples of Cu-Cr-Co/Al2O3/Al-catalyst, heat treatment which takes place in the field of microwave radiation, under identical conditions, the reaction co-deep oxidation of n- butane and CO are very active.
The observed increase in activity of the samples produced using microwave heating is probably associated with the formation on the surface of the catalysts deposited type a fine crystalline phase of active oxides metal fishing variable valence resulting from uniform sample heating when exposed to microwave radiation in a shorter period of time. This indirectly indicates a relatively large area of the peaks of the absorption maxima of hydrogen at a temperature programmed reduction of metal oxides comprising the active mass of the catalyst samples.
5. Conclusions
Thus, the results suggest that the formation of the Cu-Cr-Co/Al2O3/Al-catalysts joint deep oxidation of hydrocarbons and CO matched component composition using a heat treatment in a microwave field is acceptable for practical implementation, and from the point of view saving time and energy cost compares favorably with the traditional processes of heat.
One important factor in improving the operating performance of heterogeneous catalytic processes, the thermal activation of catalysts is carried out at the stage of their preparation, and in the immediate environment of
use. Thermal activation of the catalyst systems and methods of convection heat transfer methods is highly energy intensive and not very effective. With the increasing complexity of the structure and functionality of the catalysts, conventional methods of heat treatment are even less effective.
Results achieved by this study determines that the process formation of Cu-Cr-Co/Al2O3/Al-catalysts of deep oxidation of hydrocarbons and CO matched component composition using a heat treatment in a microwave field is acceptable for practical implementation, but in terms of saving time and energy cost it compares favorably with the traditional processes of heat.