Alkali Treatment and Cellulose Nanowhiskers Extracted from Maize Stalk Residues ()
1. Introduction
Agricultural residue is one of the largest segments of the nationwide waste problems. These large volumes of agricultural waste threaten surface water and groundwater quality in the event of waste spills, leakage from waste storage facilities, and runoff from fields on which an excessive amount of waste has been applied as fertilizer [1] . The objectives of recycling are to save resources as well as reduce the environmental impact of waste by reducing the amount of waste disposed at landfills. Waste management in South Africa faces numerous challenges due to growing population and economy, leading to increased volumes of waste generated. Farms also formed the part of waste problem which also experience major challenges in handling agricultural wastes. For example, after reaping, a farmer would either leave the residues on the land or utilize it mainly as an energy source. In many countries, animals utilize approximately 40% of maize stalk residues and the remaining crop residue is waste [2] . Many researches have investigated the use of agricultural wastes for applications in biofuel [3] [4] ; however, very little information was reported on utilization of maize as a cellulose source. The use of crop residues will also assist in the development of sustainable products with a significant added value as the results new business opportunities in the plastic of filler industrial sectors may emerge. South Africa’s Gross Domestic Product (GDP) growth rate will improve as recent market estimates that the global filler market will earn revenues of approximately US $22.4 billion in 2018 [4] . In 2010, the Asia-Pacific was the largest sales market, generating about 45% of the worldwide demand for fillers. The region was followed by Western Europe and North America. Also, global plastic production increased by 10 million tonnes (3.7%) to around 280 million tons in 2011. The use of cellulose nanowhiskers in the plastic industry is expected to continue to account for the largest share of demands. This study will contribute to the improvement in competitiveness of South Africa, thereby improving people’s lives. Not only that but it will adds value to agricultural residues through value added products.
Following the trend of previous research done, natural cellulose fibres have gained attention as the reinforcing phase in thermoplastic matrices [5] -[7] . However, such fibres are used only to a limited extent in industrial practice, which may be explained by difficulties in achieving acceptable dispersion levels. As the results, cellulose nanowhiskers are prepared from natural fibres. The reinforcing ability of the cellulose nanowhiskers lies in their high surface area and good mechanical properties [8] [9] . The tensile strength properties of whiskers are far above those of the current high-volume content reinforcements and allow the processing of the highest attainable composite strengths. Cellulose nanowhiskers can be used as reinforcing materials into polymer matrices to produce nano-composites. Biobased nanocomposites are a relatively new class of composites that exhibit ultrafine phase dimensions of 1 - 1000 nm [10] . Because of the nano-metric size effect, these composites have some unique outstanding properties with respect to their conventional micro-composite counterparts. They are considered to be one of the most important green materials for the next generation. They have the greatest market growth potential in industries, where biocompatibility and environmentally responsible design and constructions are required. The potential applications include use in automotive and construction sectors. Many researchers have investigated the use of cellulose nanowhiskers (CNW) as fillers in composites materials using natural fibres such as mulberry, coconut husk, ramie, hempand cottonas raw materials [11] -[15] . To the best of our knowledge, there is limited information on extraction of cellulose nanowhiskers from maize stalks possible for applications in green buildings and construction sector.
The extraction of cellulose nanowhiskers from biomass involves a purification step which is crucial and must be done very carefully. Chemical extraction is necessary for the development of high quality pure cellulose from biomass residues. The chemical extraction process entails alkali treatment (NaOH) followed by bleaching (NaClO2) using acetate buffer (solution of NaClO2 and glacial acetic acid), and potassium hydroxide (KOH). Glacial acetic acid is necessary to regulate the pH. Alkali treatment is performed to dissolve pectin and hemicellulose. The bleaching step removes the phenolic groups and purifies cellulose material [15] [16] . The cutting mill and the pulverizer play a crucial role in the process of mechanical extraction. The cutting mill is used for size reduction while pulverizer is essential for the removal the cell wall (lignin) to expose the microfibers from which cellulose is extracted. The aim of the current paper is to use the maize stalk to extract cellulose nanowhiskers for potential applications in green buildings in South Africa.
2. Experimental
2.1. Materials
Maize stalk was supplied by a farm in Cofimvaba in Eastern Cape, South Africa. Sodium hydroxide pellets (99.9%), potassium hydroxide (85%) and sulphuric acid (98%) were obtained from Minema Chemicals, sodium chlorite (80%) and cellulose was obtained from Sigma Aldrich. All chemicals were used as received without further purification.
2.2. Methods
2.2.1. Cellulose Extraction
Maize stalks were subjected to a cutting mill (mechanical treatment) for size reduction, operating at 1700 rpm using 2 mm sieve (Scheme 1). The resulting powder was immersed in deionized water and pulverized by super mass collider using 75% set frequency and the distance between the ceramic stones was kept at 150 μm throughout the process. The resulting suspension was filtered and dried at 50˚C overnight. Chemical treatment (NaOH, NaClO2 and KOH), was used to remove lignin; hemicellulose and extractives from maize stalk (Scheme 2). The dried maize stalk powder was subjected to alkali treatment for 1 hour at 80˚C. In this method, 1.5% NaOH aqueous solution was used and the temperature was monitored and maintained at 80˚C for an hour. The resulting maize stalks material was washed several times with deionised water until the pH was neutral. This procedure was repeated four times. Alkali treated powder was dried at 50˚C overnight. The alkali treated maize stalk was subjected to NaClO2 treatment following the same procedure as alkali treatment and finally 1.5% KOH treatment was applied to remove traces of the hemicellulose from the cellulose derived from maize stalk. The effect of chemical and mechanical treatment was investigated using XRD, FTIR, Raman spectroscopy and TGA respectively.
2.2.2. Extraction of Cellulose Nanowhiskers (CNW)
The purified cellulose derived from maize stalks was subjected to 50% H2SO4 at 40˚C for 30 min under vigorous stirring at 1000 rpm. After 30 min, the reaction was stopped by adding 500 ml of deionized water to the suspension. Refrigerated centrifuge was used to remove the acid from the suspension followed by dialysis against deionized water for three days to neutralize the pH.
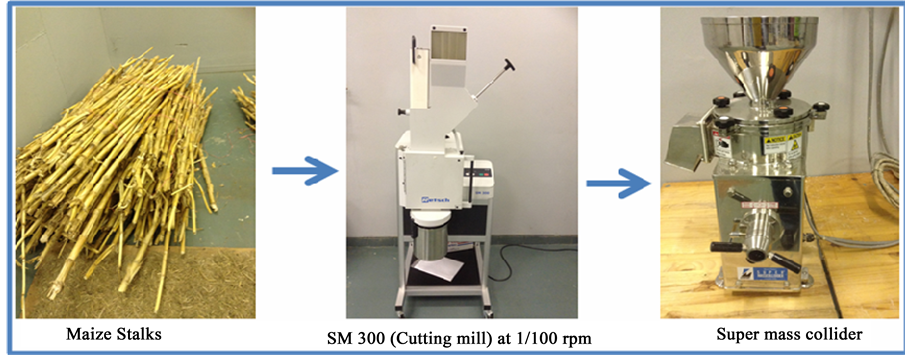
Scheme 1. Mechanical method used to synthesize cellulose nanowhiskers from maize stalks.

Scheme 2. Chemical methods used to synthesize cellulose nanowhiskers from maize stalks.
2.2.3. Chemical Composition
The lignin content of the samples was analysed by reaction with sulphuric acid using a standard method recommended in TAPPI-T222 om-88 and the hemicellulose content was obtained as described in TAPPI T19m-54.
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
SEM measurements were carried out using an FEI Quanta 200 (FEI Co., Eindhoven, The Netherlands) electron microscope and operated at an accelerating voltage of 20 kV.
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR of the samples was carried out on a Spectrum 100 FTIR (Perkin Elmer, Waltham, MA, USA). The range used was between 500 and 4000 cm−1.
2.3.3. X-Ray Diffraction (XRD)
X-ray diffraction (XRD) patterns of the samples were recorded using Philips PW 1830 X-ray diffractometer with Cu Kα radiation (λ = 0.154 nm). The crystallinity index (CI) was determined using two methods:
1) The XRD peak height method: In this method CI is calculated from the height of the 002 peak (I002) and the height of the minimum (IAM) between the 002 peak and the 001 peaks.
2) In the second method, the sample crystallinity was determined using XRD deconvolution method. This method is done by taking the amorphous and crystalline contribution to the diffraction intensity [17] .
Where I(002) is the counter reading at peak intensity at 2θ angle close to 26˚ representing crystalline material and I(AM) is the counter reading at peak intensity at a 2θ angle close to 18˚ representing amorphous material in cellulose fibres.
2.3.4. Thermo Gravimetric Analysis (TGA)
Thermo gravimetric analysis was carried out with a Perkin Elmer Pyris1 by using nitrogen as a purge gas and a heating rate of 10˚C∙min−1. The TGA programme was conditioned to ramp the temperature linearly from room temperature to 600˚C under a flow of nitrogen. The temperature of the sample was monitored and the loss of weight of the sample was expressed in terms of percentage weight loss.
2.3.5. Raman Spectroscopy (RS)
FTIR-Raman spectroscopy of cellulose extracted from maize stalkwas carried out on a Bruker FT Raman II using Intergrated air-cooled diode pumped Nd: Yag laser, 1064 nm laser and the laser power used was 500 mV at 4 cm−1 resolution. The number of scans taken were 50 and the detector utilized was liquid nitrogen (N2) cooled Ge detector.
2.3.6. Atomic Force Microscopy (AFM)
The dimensions of cellulose nanowhiskers were evaluated using atomic force microscopy (AFM) nanoscope V Microscope (Veeco Instrument Inc, Santa Babara, CA, USA) in multimode. All the samples were imaged in tapping mode. The instrument was operated at a resonance frequency of 171 kHz and a spring constant of 10 - 200 nm−1.A drop of diluted suspension of the material was deposited onto freshly cleaved mica and left to dry at room temperature for 12 hours before the analysis.
3. Results and Discussion
3.1. Chemical Composition
The chemical compositions of both wet and dry maize are represented in Table 1. Both types seemed to have relative contents of hemicellulose, lignin and cellulose approximately 30%, 17% and 50% respectively. These values and an order of chemical contents fall within an expected range when taking literature of natural fibres into consideration [18] . Drying process somehow partially damaged or/and removed lignin and hemicellulose, which crystallized more into cellulose.
![]()
Table 1. Chemical composition of untreated maize stalk (wet and dry).
![]()
Figure 1. (a) FTIR Spectra of agro-residue before and after mechanical/chemical treatment and (b) a zoomed section.
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
Figure 1 shows IR spectra of bio-residue before and after chemical/mechanical treatment. As expected, all bands revealed some of important peaks common for most natural fibres [18] [19] . For instance, the presence of hydroxyl (-OH), C-H stretching of methyl groups and C-H bending vibrations bands could be observed at 3333 cm−1, 2903 cm−1 and 1380 cm−1 respectively. The intensities of all bands increased after both mechanical and chemical treatments due to the removal of lignin and hemicellulose (Figure 1(a)), except a peak at 1740 cm−1 which is either not observed or invisible after the mechanical and chemical treated maize stalk. The peak could be certainly be related to aromatic containing groups contained in lignin and hemicellulose. Mohanty et al. [20] and Rong et al. [21] found a similar observation; however they only did chemical treatments for their studies. After KOH treatment, new functional groups (at 1746 and 1380 cm−1) were observed on the surface of purified cellulose. Fahma et al. [22] ; Mohanty et al. [20] and Rong et al. [21] in the similar study found that the functional groups plays major role in thermomechanical properties of the fibre.
3.3. Raman Spectroscopy
Raman spectroscopy evaluates the changes on the cellulose structure and measures the degree of deformation on the cellulose lattice due to sample strain/compression (Figure 2). Roman spectroscopy spectrum of purchased cellulose fibres was given for comparison. The experimental data reveal that pure cellulose material was most probably obtained after chemical and mechanical extraction of the bio-residue (Figure 2(a)). Pure cellulose is characterized by the absorption peak at 1095 (CO-stretching) and absorption peak at 1414 cm−1 which indicates the contribution of hydrogen in the cellulose lattice (HCC, HOC, HCO) (Figure 2(b)) [16] [18] [20] .
3.4. X-Ray Diffraction (XRD)
Figure 3 illustrates the XRD patterns of bio-residue before and after chemical/mechanical treatments. The diffraction lines pertain to cellulose I type as there was no doublet in the main peak at 2θ = 22.6˚ [23] . The diffractograms of both untreated and treated samples display a well-defined main peak around 2θ = 22.6˚, associated with the diffraction plane (002) of cellulose I [21] . A broad unresolved diffraction peak in the range of 13˚ - 18˚ (2θ) corresponds to (101) crystallographic planes of cellulose. In most cases when the crystalline cellulose content is high, these two peaks are more pronounced and when the fibre contains large amounts of amorphous material (such as lignin, hemicellulose, pectins and amorphous cellulose), these two diffraction peaks appear halo [24] . That may also be the case in our study and these results are in agreement with FTIR, except pulverised samples, as seen in Table 2, which is the only sample shown a decrease in crystallinity index compared to untreated. The contradiction could be explained by a destruction of long range by milling and/or partial removal of amorphous material.
![]()
Figure 2. Raman spectroscopy spectra of the purified cellulose extracted from bio-residue and purchased cellulose (for comparison).
![]()
Figure 3. XRD spectra of bio-residue before and after chemical/mechanical treatment.
![]()
Table 2. Crystallinity index of bio-residues before and after treatment (chemical/mechanical).
aCrystallinity index determined by XRD deconvolution method; bCrystallinity index determined by XRD peak height method.
3.5. Scanning Electron Microscopy (SEM)
The SEM micrographs of the mechanical and chemical treated bio-residue are illustrated in Figure 4. Untreated maize stalk showed a bundle of fibres in tacked with whitish substance on the surface relatable to non-cellulosic material such as lignin and hemicellulose (Figure 4(a)) [25] . After pulveriser, there was appearance of edges and a decrease in diameter which resulted from vigorous breakdown of the cell wall which resulted in fibrillation of fibres. Li et al. [25] obtained similar observation and related it to solubilisation of lignin and hemicellulose by alkali. In fact, KOH treatment in this study has shown an outstanding surface area (diameter ~10 μm) compared to the rest (Figure 5). This observation further explains better new functional groups and crystallinity index value observed from FTIR and XRD respectively.
3.6. Atomic Forcce Microscopy (AFM)
Figure 6 represents the micrograph of cellulose nanowhiskers (CNW) after acid hydrolysis. It is apparent from Figure 6 that further defibrillation during acid hydrolysis occurred to produce cellulose nanowhiskers (CNW) with needle-like shape. Cellulose nanowhiskers seem to be well dispersed with variable diameter ranging from 3 and 9 nm whereas length ranges from 150 - 450 nm respectively. According to the recent literature, the nanofiber could be used to bring about a high performance in different polymeric matrices [18] [28] [29] .
![]()
Figure 4. SEM images of maize stalk before and after chemical treatment.
![]()
Figure 5. SEM image of maize stalk after KOH treatment.
![]()
Figure 6. AFM images of cellulose nanowhiskers (CNW) extracted from bio-residue.
3.7. Thermo Gravimetric Analysis (TGA)
Figure 7 illustrates thermogravimetric and derivative thermogravimetry curves (DTG) behaviour of untreated and chemical/mechanical treated bio-residue. All the curves show single thermal degradation step with a char content of the untreated maize stalk highest than the rest. The highest char content could be related to lignin and hemicellulose quantity as observed from chemical composition and XRD results. Both mechanical and chemical treatments revealed an improvement in thermal stability although the pulverised sample was lowest compared to all chemically treated maize stalks. Zhang et al. [26] and Ouajai et al. [27] studied morphology of wood cellulose during milling with pan and ball mill respectively. In contrast to this study, the thermal stability of pan-milled and ball milled cellulose was gradually decreased with the milling cycles and it was attributed to the reduction of crystalline cellulose and increase of amorphous cellulose which has a lower thermal stability. However, there was no experimental evidence to support the rational. By the way, in this study, the increased
![]()
Figure 7. TGA and DTG profiles of bio-residue at different treatment stages.
thermal stability after milling could have emanated from the partial removal of lignin and hemicellulose, as observed from chemical composition and FTIR, sincelignin and hemicellulos are well known catalyst of thermal degradations of natural fibres. Thermal stability of NaOH and NaClO2 treated maize stalk is relatively similar, whereas KOH treated maize stalk has shown a significant decrease. This suggests that lignin and hemicellulose were well removed in the treatments and somehow cellulose was partially depolymerized after KOH treatment.
4. Conclusions
・ Chemical method, alkali, bleaching and potassium hydroxide treatment were used and the experimental results showed that lignin and hemicellulose were removed significantly from the bio-residues. The mechanical treatment (pulverizer) also revealed a partial removal of non-cellulosic materials from the raw material.
・ All the treated maize stalks showed a clear single thermal degradation step and char lower than the untreated. The results correspond well with chemical composition and XRD results. Both mechanical and chemical treatments revealed an improvement in thermal stability although the pulverised sample was lowest compared to all chemically treated maize stalks.
・ High quality cellulose was extracted from maize stalk residues by chemical method.
・ Acid hydrolysis of cellulose derived from maize stalk residues resulted to high dispersed needle-like shapecellulose nanowhiskers (CNW).
・ The resulting CNW could find potential applications as reinforcement in biopolymers in the Republic of South Africa.
Acknowledgements
This paper forms part of a research project, “Greener Cities in South Africa”, funded by the Green Fund, an environmental finance mechanism implemented by the Development Bank of Southern Africa (DBSA) on behalf of the Department of Environmental Affairs (DEA), and the university of Zululand South Africa. Opinions expressed and conclusions arrived at, are those of the authors.
NOTES
*Corresponding author.