Improve Water Quality at the El-Rahawy Drain and the Rosetta Branch, Egypt ()
1. Introduction
Agricultural drains form major sources of pollution along the Nile River. From Aswan Dam to the Mediterranean Sea, the Nile River receives wastewater discharges from 74 agricultural drains [1] [2] . Major pollutants in agricultural drains include salts, nutrients (phosphorus and nitrogen), pesticide residues from irrigated fields, pathogens from domestic wastewater, and toxic organic and inorganic pollutants from domestic and industrial sources [3] . The data indicate that all drains located along the Nile River exceed the consent standards set by Law 48/1982 in one or more of the parameters [3] . The river separates at Cairo into two branches, Rosetta and Damietta branches, which form the Nile delta. The Rosetta branch is about 239 km (148.50 miles) in length. The Rosetta branch daily receives more than 3 million cubic meters (Mm3) of untreated and partially treated industrial and domestic wastewaters, in addition to receiving agricultural drainage water [4] [5] . The El-Rahawy drain is a major cause of pollution at the Rosetta branch [4] . It receives water from Zenen and Abu-Rawash wastewater treatment plants (WWTPs), and from agricultural drainage [4] [5] . The Rosetta branch daily receives approximately 501,926,900 gallons (1,900,000 m3) of drainage water from the El-Rahawy drain. The Abu-Rawash WWTP is one of the largest wastewater treatment plants in Egypt and considers the main cause of water quality degradation at the El-Rahawy drain and the Rosetta branch. The Abu-Rawash plant provides only a primary treatment and can handle about 437,006,400 gal/day (1,200,000 m3/day) of peak flow [2] [6] . The plant daily receives more than 383,049,480 gallons (1,450,000 m3) of raw wastewater, which clearly exceeded the design capacity [7] . Therefore, excess sewage is bypassed and discharged directly to the El-Rahawy drain without prior treatment, which causes an increase in pollution levels at the El-Rahawy drain and the Rosetta branch [8] . The average concentrations of total suspended solids (TSS), total organic carbon (TOC), dissolved oxygen (DO), biological oxygen demand (BOD), total dissolved solids (TDS), and chemical oxygen demand (COD) in the El- Rahawy drain were 159.25, 9.20, 1.45, 146.70, 720, and 270 mg/L, respectively [9] . The average concentrations of turbidity, TSS, TOC, DO, BOD, TDS, and COD in the Abu-Rawash WWTP effluent were 19, 92.40, 9.70, 0.955, 99, 937, and 209.50 mg/L, respectively [9] . The effluent water quality does not meet the applicable water quality standards specified in Egyptian law 48/1982 due to providing only primary treatment. The water quality standards specified in Egyptian law 48/1982 for COD, BOD, TDS, DO, turbidity, and TSS are ≤30 mg/L, ≤20 mg/L, ≤500 mg/L, ≥5 mg/L, ≤50 mg/L, and ≤20 mg/L, respectively [9] .
Several studies have been conducted to address water quality at the El-Rahawy drain. In their study, Abdel- Satar and Elewa (2001) collected water samples from the El-Rahaway drain and the Rosetta branch; after analyzing the collected water samples for different parameters, the authors observed high levels of physicochemical parameters in the Rosetta branch at the discharge point of the El-Rahawy drain [10] . Abdo (2002) collected water samples from different points along the Rosetta branch concluded that the El-Rahawy drain is the main cause of water quality degradation at the Rosetta branch [11] . Badr et al. (2006) and Elewa et al. (2009) also observed high levels of physicochemical parameters at the discharge points of the El-Rahawy drain [4] [12] . They concluded that the agricultural and domestic wastewaters are the major sources of water pollution at the El-Rahawy drain. In their study, Moustafa et al. (2010) and Ezzat et al. (2012) collected water samples from different points along the branch for analysis [13] [14] . Because results showed low concentration of DO and high concentrations of turbidity, TDS, COD, BOD, ammonia (NH3), bicarbonate (HCO3), and total alkalinity at the discharge point of the El-Rahawy drain, the researchers concluded that the Rosetta branch water quality is adversely affected by receiving discharge from the El-Rahawy drain. Mostafa (2014) conducted a study to assess and manage water quality at the Rosetta branch. He collected water samples from point sources discharging to the El-Rahawy drain for analysis. The author concluded that the Abu-Rawash WWTP comprises the major source of pollutants along the El-Rahawy drain and the Rosetta branch [9] . In order to manage the effluent water quality from the Abu-Rawash WWTP, Mostafa (2014) conducted a research to evaluate the effectiveness of aluminum chloride (AlCl3) in wastewater treatment [9] . Results showed that the AlCl3 is more efficient in wastewater treatment than ferric chloride, ferric sulfate, and ferrous sulfate. Results also showed that the optimal pH values for the elimination of the COD, TSS, BOD, and turbidity ranged from 6.10 to 6.20 for the AlCl3. Carbon dioxide (CO2) was injected into the sewage sample in order to reduce pH value.
2. Materials and Methods
This research involved attempting to determine the optimal dose of AlCl3 to reach an acceptable treatment at the Abu-Rawash WWTP. First, five wastewater samples were collected in plastic containers after screening and grit removal. At pH 6.14, eleven different doses of aluminum chloride were applied into the beakers, each containing 1.0 L of sewage. A blank jar with no coagulant was also prepared. Jars were then placed in a stirrer with paddles. The mixing speed was set at 150 rpm for one minute. Then, the stirring speed reduced to 30 rpm for 10 minutes. Last, samples were left to settle for 45 minutes under quiescent conditions [9] . The resulting liquid samples were then analyzed for pH, TOC, turbidity, TSS, TDS, COD, and BOD. The WTW multi 340i meter was used to measure the pH and DO values in the field. The multi meter was calibrated using buffer solutions with pH values of 4.0, 7.0, and 10. The meter automatically adjusts DO. The TDS concentration was also measured in the field using the HM digital TDS meter. The accuracy of measurement was assessed by analyzing a sample with known concentration. Analysis of the other parameters took place in Egyptian Housing Building Research Center (HBRC) laboratory, located in Cairo city. The samples were analyzed for TOC, turbidity, TSS, COD, and BOD according to the standard methods for wastewater analysis [15] . The turbidity meter enabled measurement of turbidity. A Shimadzu TOC-4200 analyzer was used to measure the TOC concentration. The accuracy of measurement was assessed by analyzing samples with known concentration. Use of 5-day BOD Test 5210B enabled determination of the BOD concentration in the samples. The 5-day BOD Test 5210B was used in the determination of the BOD concentration in wastewater samples. For quality control purposes, seed control samples were tested after 5 days of incubation; in addition the dilution water and the glucose-glutamic acid solution were tested and compared with the acceptable limits. The TSS concentration was measured using the test method 2540D. The accuracy of measurement was assessed by analyzing 20% of the total number of sample. The closed reflux, titrimetric method 5220C was used in the determination of the COD concentration in the samples. The accuracy of measurement was assessed by analyzing a sample with known concentration [9] .
Then, the removal efficiency was calculated for each parameter using the following formula:
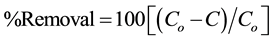
where: Co = parameter concentration at the blank sample,
C = parameter concentration after coagulation treatment.
Another approach involves discharging flow from Al-Buhairi Water Canal (flows perpendicular to the El- Rahawy drain and parallel to the Rosetta branch) to the El-Rahawy drain in order to reduce pollutant concen- trations at the El-Rahawy drain. This approach could be easily applied without the need for further modifications; an existing gate that connects the Al-Buhairi Water Canal with the El-Rahawy drain.
Mass balances were used to estimate the concentrations of different parameters at the El-Rahawy drain after effluent water quality was improved at the Abu-Rawash WWTP and discharging water from the Al-Buhairi Water Canal. The river pollutant (RP) modeling was then used to study the effect of improving water quality at the El-Rahawy drain on the Rosetta branch water quality [9] .
3. Results and Discussion
A series of jar tests were used to determine the optimal dose of AlCl3 to reach an acceptable treatment. At pH 6.14, eleven different doses of AlCl3 were applied into the beakers. To reach an acceptable treatment with the least possible cost, very low doses of aluminum chloride were selected. The doses were 0.40, 1.0, 1.50, 2.0, 2.50, 3.0, 3.50, 5.0, 6.50, 8.0, and 10.0 mg/L. Samples were analyzed for COD, BOD, TSS, TDS, TOC, and turbidity, and the removal efficiencies are presented in Figure 1. To reach an acceptable treatment with the least possible cost, a dose of 2.0 mg of aluminum chloride for each liter of wastewater was selected.
The initial COD concentration was 324.8 mg/L. Application of 2.0 mg/L of aluminum chloride at pH 6.14 reduced the COD concentration at the effluent from 324.80 mg/L to 106 mg/L. The maximum removal efficiency of COD was 67.60%, whereas it was limited to 38.0% for the blank sample. For the same dose, the BOD, TOC, TDS, TSS, and turbidity reached maximum removal efficiencies of 66.0%, 61.80%, 64.60%, 82.20%, and 80.10%, respectively (see Figure 2).
The El-Rahawy drain receives about 383,049,500 gal/day (1,450,000 m3/day) of primary treated wastewater from the Abu-Rawash WWTP and about 118,877,400 gal/day (450,000 m3/day) of secondary treated wastewater from the Zenen WWTP. The average TOC, DO, TDS, TSS, BOD, and COD concentrations at the effluent of the Zenen WWTP were recorded to be 1.50, 4.50, 412.0, 30.0, 34.50, and 70.0 mg/L, respectively. And the average pH value was recorded to be 7.4. After applying 2.0 mg/L of aluminum chloride at pH 6.14, the average concentrations of TOC, DO, TDS, TSS, BOD, and COD at the effluent of Abu-Rawash WWTP were recorded to be 5.60, 0.92, 355, 41.50, 57.60, and 106 mg/L, respectively. Application of 2.0 mg/L of aluminum chloride at pH 6.14 also reduced the total phosphorus (TP) concentration at the effluent from 3.0 mg/L to 1.50 mg/L.
The effluents from Abu-Rawash and Zenen WWTPs travel about 16.5 km and 26.5 km, respectively, to reach the El-Rahawy drain. So, the exponential equation 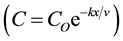 was used to calculate the concentration of different parameters directly before pouring to the El-Rahawy drain. The decay rate was assumed 0.108 day−1 at
was used to calculate the concentration of different parameters directly before pouring to the El-Rahawy drain. The decay rate was assumed 0.108 day−1 at
![]()
Figure 1. Effect of carbon dioxide and aluminum chloride doses on BOD, COD, TSS, and turbidity removal efficiencies.
![]()
Figure 2. Removal efficiencies when applying 2 mg/L aluminum chloride at pH 6.14.
21.7˚C [9] . The concentrations of DO, TOC, TSS, BOD, TDS, and COD at Abu-Rawash WWTP effluent directly before pouring to the El-Rahawy drain were 0.89, 5.40, 40.0, 55.50, 388, and 102.10 mg/L, respectively. The concentrations of DO, TOC, TSS, BOD, TDS, and COD at Zenen WWTP effluent directly before pouring to the El-Rahawy drain were 4.24, 1.41, 28.25, 32.50, 388, and 66 mg/L, respectively.
Mass balances were involved to estimate the flow and the concentrations of different parameters at the El- Rahawy drain after the effluents from Abu-Rawash and Zenen WWTPs were mixed, as shown in the equations below. Improving effluent water quality at the Abu-Rawash WWTP reduced the BOD concentration at the El- Rahawy drain from 146.70 mg/L to 50.0 mg/L. This modification also increased the level of DO by 17.24%, a reduced the levels of COD, TOC, TSS, and TDS by 65.20%, 51.52%, 64.0%, and 51.0%, respectively.
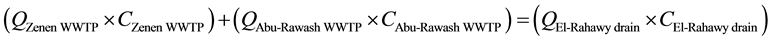 (1)
(1)
For COD:
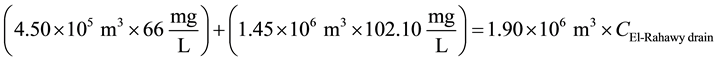
\ 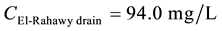
For BOD:
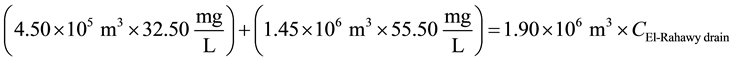
\ 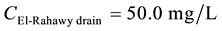
For TSS:
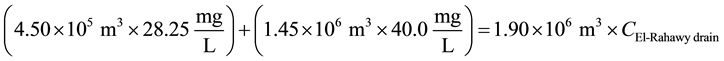
\ 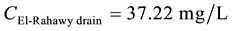
For DO:
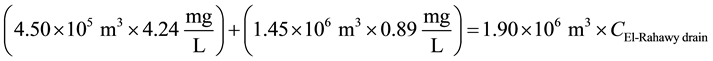
\ 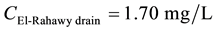
For TDS:
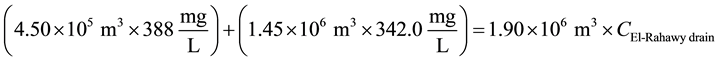
\ 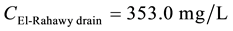
For TOC:
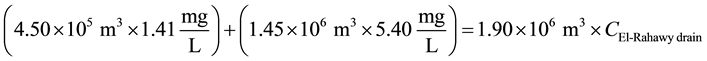
\ ![]()
For pH:
![]() (2)
(2)
![]()
\ ![]()
The second approach involves discharging flow from the Al-Buhairi Water Canal to the El-Rahawy drain. The concentrations of TOC, TDS, COD, BOD, TSS, and DO in the Al-Buhairi Water Canal were 0.72, 189.6, 12.50, 4.80, 22.80, and 6.13 mg/L, respectively. Mass balances were also used to estimate the concentrations of different parameters at the El-Rahawy drain after discharging water from the Al-Buhairi Water Canal, as shown in the equations below. Discharging 1.0 million m3 (264,172,000 gallons) of water daily from the Al-Buhairi Water Canal to the El-Rahawy drain reduced the COD concentration at the El-Rahawy drain from 94.0 mg/L to 65.60 mg/L and increased the DO concentration from 1.70 mg/L to 3.22 mg/L, which in turn will lead to improved water quality in the Rosetta branch. This modification also reduced the concentrations of TOC, TDS, TSS, and BOD by 28.92%, 15.97%, 13.35%, and 31.04%, respectively.
![]() (3)
(3)
For COD:
![]()
\ ![]()
For BOD:
![]()
\ ![]()
For TSS:
![]()
\ ![]()
For DO:
![]()
\ ![]()
For TDS:
![]()
\ ![]()
For TOC:
![]()
\ ![]()
For pH:
![]() (4)
(4)
![]()
\ ![]()
Applying the two approaches together are projected to increase the DO concentration at the El-Rahawy drain by approximately 122%, and will decrease the TDS, TOC, BOD, TSS, and COD concentrations by about 58.80%, 65.54%, 76.50%, 79.74%, and 75.70%, respectively. Consequently, applying these approaches will significantly improve water quality at the El-Rahawy drain.
3.1. Economical Study
Coagulant unit, pH controller, and carbon dioxide unit are needed for treating wastewater by using coagulants. Assume that the effective dose of AlCl3 at pH 6.14 is 2 mg/L. The cost of AlCl3 and CO2 to treat one cubic meter of wastewater at pH 6.14 is 0.0034 Egyptian Pounds (EGP). This solution can be applied as a temporary solution until the secondary treatment units at the Abu-Rawash WWTP begin working. The secondary treatment units are expected to begin working after 9 years. Equations below show the total cost of applying the proposed solution for nine years period.
Total cost per day = cost of AlCl3 and CO2 + cost of coagulant unit + cost of carbon dioxide unit + cost of pH controller
Cost of AlCl3 and CO2, EGP/day = flow (m3/day) × [AlCl3 cost (EGP/m3) + CO2 cost (EGP/m³)] = 1,450,000 m3/day × [0.0026 EGP/m3 + 0.0008 EGP/m³] ~ 4930 EGP/day ~ $632/day
Total cost per day = 4930 EGP/day × 365 days × 9 years + 300,000 EGP + 200,000 EGP + 21,000 EGP = 16,700,000 EGP/10 years = $2,143,000/10 years
3.2. River Pollutant (RP) Modeling
The river pollutant (RP) modeling was used to predict the change in the Rosetta branch water quality after improving water quality at the El-Rahawy drain. The water quality standard specified in EPA and Egyptian law 48/1982 for COD is ≤ 10.0 mg/L [16] [17] .
The COD concentration downstream the El-Rahawy drain is expected to decrease from 31.10 mg/L to 16.50 mg/L after improving water quality at the El-Rahawy drain, as shown in Figure 3. Although the COD concentration at the Rosetta branch is expected to significantly decrease after applying the proposed scenario, but still did not meet the standards (see Figure 3). Applying the proposed solution will significantly decrease the negative affect of the El-Rahawy drain in the Rosetta branch water quality because the COD concentration downstream of the El-Rahawy drain will become very close to acceptable levels after improving water quality at this drain. The water quality standard specified in EPA and Egyptian law 48/1982 for TSS is ≤20.0 mg/L [16] [17] . Figure 4 shows that the TSS concentration upstream of the El-Rahawy drain is clearly exceeding the water quality standards. The TSS concentration downstream the El-Rahawy drain is expected to decrease from 58 mg/L to 47.77 mg/L after applying the proposed scenario (see Figure 4). The TSS concentration along the study area is also expected to decrease by about 17.60% after improving the water quality at the El-Rahawy drain. The TDS concentration upstream of the El-Rahawy drain was approximately 230 mg/L, which is clearly within the 500 mg/L maximum limit specified in Egyptian and EPA standards [16] [17] . The TDS concentration downstream of the El-Rahawy drain is expected to decrease significantly from 277.5 mg/L to 237.9 mg/L after im-
![]()
Figure 3. COD concentration along the Rosetta branch for current situation and proposed solution.
![]()
Figure 4. TSS concentration along the Rosetta branch for current situation and proposed solution.
proving water quality at the El-Rahawy drain (see Figure 5). The BOD concentration upstream of the El-Ra- hawy drain was within the limits specified in Egyptian law 48/1982 and EPA standards is ≤6.0 mg/L [16] [17] . In the current situation, the BOD concentrations downstream of the El-Rahawy drain was about 15.95 mg/L. This value clearly exceeded the water quality standards, as shown in Figure 6. The BOD concentration downstream of the El-Rahawy drain is expected to significantly decrease from 15.95 mg/L to 8.40 mg/L after improving water quality at the El-Rahawy drain. Applying the proposed solution is expected to decrease the BOD concentration at the Rosetta branch downstream of the El-Rahawy drain by about 46.8%. Consequently, if the proposed solution is applied, the BOD concentration downstream of the El-Rahawy drain will become very close to acceptable levels, which leads to improving the water quality at the Rosetta branch.
The TOC concentration upstream of the El-Rahawy drain was approximately 0.6 mg/L, which clearly is within the 3.0 mg/L maximum value specified in Egyptian law 48/1982 [16] . In the two cases, the TOC concentration along the study area was also within the permissible limits. The TOC concentration downstream of the El-Rahawy drain is expected to decrease significantly from 1.52 mg/L to 0.86 mg/L after improving the water quality at the El-Rahawy drain (see Figure 7). In addition, the TOC concentration along the study area is expected to decrease by about 43.40% after applying the proposed scenario. The pH value downstream of the El-Rahawy drain is expected to decrease from 8.1 mg/L to 7.31 mg/L after improving water quality at the El-Rahawy drain, as shown in Figure 8. For the current situation and the proposed solution, the pH value along the Rosetta branch agrees with water quality standards specified in EPA and Egyptian law 48/1982 (7.0 to 8.5) [16] [17] . The major cause of pH decline involves the use of carbon dioxide in sewage treatment. The DO concentration specified in Egyptian law 48/1982 and EPA standards is ≥4.0 mg/L [16] [17] . In the two cases, the DO concentration along the Rosetta branch was within the standard limits in all areas but downstream of the Tala drain (see Figure 9). A slight increase in dissolved oxygen concentration is expected to occur after applying the proposed scenario, due to increasing the dissolved oxygen mass loading at the El-Rahawy drain.
![]()
Figure 5. TDS concentration along the Rosetta branch for current situation and proposed solution.
![]()
Figure 6. BOD concentration along the Rosetta branch for current situation and proposed solution.
![]()
Figure 7. TOC concentration along the Rosetta branch for current situation and proposed solution.
![]()
Figure 8. pH value along the Rosetta branch for current situation and proposed solution.
![]()
Figure 9. DO concentration along the Rosetta branch for current situation and proposed solution.
4. Conclusions
Application of 2.0 mg/L of aluminum chloride and injecting CO2 caused reductions in TP, TDS, TOC, BOD, TSS, COD, and turbidity reaching 50.0%, 64.60%, 61.80%, 66.0%, 82.20%, 80.10%, and 79.60%, respectively. Improving effluent water quality at the Abu-Rawash WWTP increased the level of DO by 17.24% at the El- Rahawy drain, and reduced the levels of BOD, COD, TOC, TSS, and TDS by 65.92%, 65.20%, 51.52%, 64.0%, and 51.0%, respectively. The total cost of applying the proposed solution for nine years period is about 16,700,000 EGP ($2,143,000). This solution can be applied as a temporary solution until the secondary treatment units at the Abu-Rawash WWTP begin working. The total cost will decrease significantly if the units begin working before nine years.
Results also showed that discharging 1.0 million m3 of water daily from the Al-Buhairi Water Canal to the El-Rahawy drain will reduce the COD concentration at the El-Rahawy drain from 94.0 mg/L to 65.60 mg/L and
will increase the DO concentration from 1.70 mg/L to 3.22 mg/L. Applying this approach will also reduce the BOD, TSS, TDS, and TOC concentrations at the El-Rahawy drain by about 31.0%, 13.35%, 15.97%, and 28.92% respectively. Applying the two approaches together is projected to increase the DO concentration at the El-Ra- hawy drain by approximately 122%, and will decrease the TDS, TOC, BOD, TSS, and COD concentrations by about 58.80%, 65.54%, 76.50%, 79.74%, and 75.70%, respectively.
The RP modeling shows that the concentrations of COD, TDS, BOD, TSS, TOC, and chlorides along the Rosetta branch are expected to decrease after the Abu-Rawash WWTP effluent is improved and after water is discharged from the Al-Buhairi Water Canal to the El-Rahawy drain. In addition, a slight increase in dissolved oxygen concentration is expected to occur.
After the revolution of January 25, 2011 took place, the Egyptian government started to pay more attention to water issues by allocating more funds toward the water and wastewater sector. Consequently, applying the proposed solution was currently possible, especially because of its cost-effectiveness which exceeded that of the other proposed solutions such as changing the effluent path of the Abu-Rawash WWTP to the desert.
Acknowledgements
This research was supported by the Department of Civil, Construction, and Environmental Engineering at the University of Alabama at Birmingham. The authors also thank the Egyptian Housing Building Research Center for their help in collecting samples and performing the chemical analyses.
NOTES
*Corresponding author.