1. Introduction
Manganese is a transition metal and has many oxidation states (+2, +3, +4) and can give many phases of manganese oxides like MnO, MnO2, Mn2O3, Mn3O4 and Mn5O8. For that reason, manganese has attracted many research efforts [1] . Manganese oxides are non-toxic, abundant and cost-effective and have a wide range of technical applications, including catalytic [2] , electrochemical [3] , magnetic [4] , optical [5] and electro catalytic biosensors [6] . MnO has a so-called rocksalt structure which can be considered as the insertion of two fcc lattices. The other common manganese oxides, mentioned above, have manganese in higher oxidation states: Mn3O4, Mn2O3 and MnO2. The first one known as the mineral hausmannite at ambient temperature has a distorted spinel
structure and can be represented by the formula 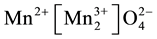 [7] . Two polymorphs of this oxide exist,
[7] . Two polymorphs of this oxide exist,
named α and β. Also, there are two polymorphs of Mn2O3 known as minerals α-kurnakite and β-kurnakite/bix- byite, respectively [8] . The most stable and common among manganese dioxides in nature is the mineral pyrolusite β-MnO2 that has a simple tetragonal rutile structure [9] . In early studies some mineral Mn oxide specimens have been analyzed [10] - [12] and morphological analysis of some powdered manganese oxides obtained from different precursors has been accomplished [13] - [15] . According to [13] , a Mn3O4 phase, prepared using different precursors, had a very broad particle size distribution ranging from 10 nm to 100 nm. Some cubic particles were observed, but many particles did not exhibit a well-defined shape. Mn3O4 particles prepared from MnO2 had a tetragonally distorted cubic shape with a size of about 25 to 40 nm. Recently, several studies on the preparation of MnO, Mn2O3 and Mn3O4 phases of nanosize dimensions were performed [16] - [21] . Generally, depending on the precursor, a variety of nanostructures were registered ranging from polyhedrons and globular structures to nanorod structures. Cubic nanoparticles of MnO (28 nm) and a-Mn2O3 (50 nm) were synthesized via manganese oxalate precursor and under specific reaction conditions [16] . In particular, Mn2O3 has attracted much interest as an anode material in the lithium ion battery and can also be used for preparing soft magnetic materials such as manganese zinc ferrite [22] . Different types of nanostructures of Mn2O3, such as three-di- mensional (3D) sponge-like porous hollow nanostructures, spheres, dumbbell, and peanut shaped microstructure, have already been reported [23] . Mn2O3 nanoparticles were synthesized from Mn (III) acetylacetonate by Mohseni et al. [24] , and their crystallite size was found to be 70 nm. Another work was done by Javed et al. [25] on Mn2O3 nanostructures―prepared by free template hydrothermal route―and the direct band gap was found to be around 1.2 eV.
This work presents the preparation of Mn2O3 nanoparticles using the co-precipitation method. The sample was characterized by XRD and TEM techniques. The elemental composition was determined by PIXE and RBS techniques. The energy gap was calculated via UV-spectroscopy. The functional groups of the samples were detected by FTIR. The magnetic properties were studied using VSM and EPR techniques.
2. Experimental Techniques
Nano-sized Mn2O3 was prepared by using Co-precipitation method, a stock solution of 0.2 M concentration of MnCl2 and an alkali solution (pH 13.8) of 4.0 M sodium hydroxide (NaOH). The reaction is fundamentally performed with stirring by mixing the stock solution of MnCl2, the alkali solution of NaOH and a certain amount of purified water. The precipitation occurred at 60˚C for 2 h after the mixing of the two solutions. The products obtained by filtration were washed with purified water and then dried at 70˚C for 18 h in air. The dried ingots were heated at 500˚C for 6 h.
The structure of Mn2O3 has been studied by means of the X-ray powder diffraction analysis using Shimadzu 7000 powder diffractometer with CuKα radiation (λ = 1.5406 Å) in the range 10˚ ≤ 2θ ≤ 80˚. The X-ray tube used was a copper tube operating at 30 kV and 30 mA. About 0.6 g of the sample was grinded very well to be in the form of fine grains powder that was placed in a holder of plate-like shaped, where the distribution of the crystallites orientations are nearly continuous.
The TEM micrograph for the nanoparticles was taken from Jeol transmission electron microscope JEM that operates at 80 kV providing magnification from 100× to 250,000× and a resolution of 0.2 nm.
The elemental composition of the Mn2O3 nanoparticles was measured by PIXE technique using the 1.7 MV tandem accelerator of the Lebanese Atomic Energy Commission [26] . 3 MeV proton beam was delivered to the sample with 1 μC of fluence. For ion beam analysis, a mass of 0.3 g of the sample was grinded very well for the powder to be homogenous and hence a thick target pellet of approximate dimensions 1 × 1 × 0.2 cm3 was formed. The real stoichiometric amounts of the elements were calculated using the following equation:
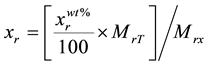 (1)
(1)
where  is the real element-content as wt%, MrT is the total molecular weight and Mrx is the molecular weight of the element. Using the same instrument, elastic backscattering measurements were taken under normal incident beam. A partially depleted PIPS (Passivity Implanted Planar Silicon) detector from Canberra, with 14 keV energy resolutions and 25 mm2 active areas, detected the backscattered particles of the 3 MeV proton beam, at a scattering angle θ of 165˚ and solid angle of 5.45 × 10−3 sr.
is the real element-content as wt%, MrT is the total molecular weight and Mrx is the molecular weight of the element. Using the same instrument, elastic backscattering measurements were taken under normal incident beam. A partially depleted PIPS (Passivity Implanted Planar Silicon) detector from Canberra, with 14 keV energy resolutions and 25 mm2 active areas, detected the backscattered particles of the 3 MeV proton beam, at a scattering angle θ of 165˚ and solid angle of 5.45 × 10−3 sr.
The FTIR analysis was carried by FTIR 8400S Shimadzu that relies on Michelson interferometer and combined with a high performance FTIR software to analyze samples easily and securely.
The optical properties were studied by mixing 0.05 g of Mn2O3 nanoparticles with 60 ml of ethanol in diluted form, ultrasonication for 6 min and then using the NIR spectrophotometer V-670 in the range 350 nm - 800 nm.
The magnetic study was conducted with Vibrating Sample Magnetometer (Lake Shore VSM 7410) with a moment measurement range of 3.1 × 10−6 emu to 1000 emu and 1% field accuracy in gauss. Spectrum of Mn2O3 sample was recorded by EPR technique using Bruker Elexsys 500 spectrometer where 0.1 g powder is mounted in a quartz tube of length 20 cm and diameter 2 mm in the middle of the cavity, where the magnetic component of the microwave power has a maximum and is oriented perpendicularly to the static field. The EPR spectra of the sample were recorded using temperature controller Bruker ER 4131.
3. Results and Discussions
The XRD diffraction spectrum for Mn2O3 nanoparticles is shown in Figure 1. In the XRD spectrum, the peaks observed at 23.1˚, 32.9˚, 38.2˚, 45.0˚, 49.3˚, 55.1˚ and 65.7˚ correspond to the planes (211), (222), (400), (332), (431), (440) and (622) of Mn2O3 nanoparticles. Compared to the standard peaks of pure Mn2O3 (JCPDS 41-1442), the hkl planes are well-indexed to a cubic phase with lattice parameter a = 9.4232Å, which was calculated using the combined formula of Bragg and the interplanar distance of the cubic structure as in Equation (2),
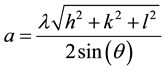 (2)
(2)
The result is in good agreement with JCPDS (card No. 89-4836) and the results reported by Thota et al. [27] .
The average crystallite size was estimated using Debye-Scherer’s formula:
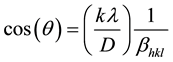 (3)
(3)
![]()
Figure 1. XRD spectrum for Mn2O3 (10˚ < 2θ < 80˚).
where D is the crystallite size, K is the shape factor (0.9), λ is the wavelength of CuKα (1.5406 Å) , βhkl is the instrument broadening width with instrumental correction at half size and θ is the peak position. A plot is drawn with 1/βhkl on the x-axis and cos(θ) on the y-axis as shown in Figure 2. D is calculated from the slope of the line of best fitting, and was found to be 17.3 nm.
TEM micrograph is taken for the Mn2O3 nanoparticles with 7500× magnification with 100 nm scale and it is shown in Figure 3. The shape of the particles is spherical. Their average crystallite size was measured and found to be 19.1 nm and this result confirms the one obtained from XRD with an acceptable difference of 1.8 nm. This result was consistent with the one obtained by Nathan et al. [28] .
The real elemental-contents of the prepared sample were determined by using the ion beam analysis. Proton induced X-ray emission in combination with backscattering spectrometry were applied for analyzing Mn2O3
![]()
Figure 2. Plot of cos(θ) vs. 1/β in Debye?Scherrer’s method for Mn2O3.
![]()
Figure 3. TEM image for Mn2O3 nanoparticles.
nanoparticles. In PIXE technique, the element Mn was identified using Kα and Kβ X ray lines, as shown in Figure 4, while oxygen is invisible with respect to PIXE since it detects only elements with Z > 10. No contaminations were observed in the spectrum which verifies the high purity of the nanoparticles. The weight percentages of Mn and O were measured, the stoichiometry was calculated using Equation (1) and the results are listed in Table 1.
Rutherford backscattering spectrometry (RBS) represents an alternative technique for an accurate determination of O-content. Thus, the O-content was determined from the 3 MeV H+ backscattering spectrum by fitting non-Rutherford backscattering cross section for O [29] in the SIMNRA simulations and shown in Figure 5. The results of the RBS techniques are also listed in Table 1. It is clearly observed, from the error %, that RBS was more accurate in the determination of the oxygen concentration. However the determined concentration for both elements in the two techniques is very close to the nominal values and to each other with an error difference of 2.81%.
![]()
Figure 4. The plots of the counts as a function of energy (eV) for Mn2O3 nanoparticles showing the emission peaks; MnKα and MnKβ.
![]()
Figure 5. RBS spectrum for Mn2O3 nanoparticles.
![]()
Table 1. Nominal, PIXE and RBS weight percentages of Mn and O contents in addition to the stoichiometric ratios of the two elements.
Optical study of Mn2O3 nanoparticles was performed by using the UV-visible optical spectroscopy in the range (350 nm - 800 nm) showing a noticeable peak at 648.41 nm and a small peak at 375.77 nm as shown in Figure 6. The smaller peak might be due to ethanol solution where the nanoparticles are mixed and the higher peak is due to Mn2O3 nanoparticles. It can be seen that the prepared sample absorbs photons strongly in the visible region (400 nm - 800 nm) that arises due to charge-transfer transition in the manganese oxide nanostructures [30] [31] . The absorption is more than 85% between 500 nm and 800 nm where it increases abruptly at 596nm and decreases at 668 nm. Based on the above result, the Mn2O3 nanoparticles can be used in applications where the absorption of visible light is required such that solar cells and photo-catalysts [25] .
The energy band gap of the direct transition (Eg) of the sample was calculated using the Tauc and Davis-Mott models expressed in a relation between the absorption coefficient (α) and the energy of the photon (hv) as follows,
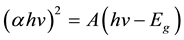 (4)
(4)
where α = 2.303 A/L, A is the absorbance of the sample and L is the path length (10 mm). The energy band gap Eg was estimated using the intercept of the linear portion of the curve (αhv)2 vs. (hv) to y = 0 as shown in the inset of Figure 6 and was found to be 1.24 eV. This value is almost close to the value reported in Mn2O3 nanorods by Rahaman et al. [32] and Mn2O3 nanostructures prepared by hydrothermal synthesis [25] .
FTIR spectrum of Mn2O3 nanoparticles was studied between 400 cm−1 and 4000 cm−1 as shown in Figure 7. The absorption peaks 620.10 cm−1 and 540.09 cm−1 correspond to the stretching vibration of Mn?O and Mn?O?Mn bonds indicating the formation of Mn2O3 [33] . 3448.84 cm−1 peak resulting from O-H stretching vibration of water molecule that remained in the powder during the preparation technique.
Magnetic characterization of Mn2O3 nanoparticles were studied by tracing room temperature magnetic hysteresis loop, as shown in Figure 8, that illustrates the variation of the magnetization (M) in emu/g as a function of an applied magnetic field (H) in the range 20,000 G ≤ H ≤ 20,000 G. It shows antiferromagnetic behavior like a bulk material. This behavior is similar to that reported by Pugazhvadivu et al [34] and contradicted that reported by Javed et al. [25] . They showed a paramagnetic behavior at room temperature and antiferromagnetic at TN ≈ 83 K. This contradiction in the results is attributed to the difference in the preparation method, crystal structure and crystalline size.
The saturation magnetization Ms of Mn2O3 nanoparticles was deduced from the extrapolation of the M vs.1/H curves to 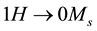 was calculated and found to be 2.642 emu/g. The coercivity (Hc) and the retentivity (Mr) were also measured and listed in Table 2.
was calculated and found to be 2.642 emu/g. The coercivity (Hc) and the retentivity (Mr) were also measured and listed in Table 2.
EPR analysis was performed at room temperature for Mn2O3 NPs, where Figure 9 shows the variation of the magnetic field versus the intensity and its inset shows the variation of the g-factor versus the intensity. The g-factor is calculated from the graph and found to be 1.985, and the magnetic field shift occurred at Bo = 350.5 mT. These values are quiet similar to those reported by Pugazhvadivu et al [34] for Mn2O3 nanoparticles doped with cobalt in small concentrations. It can be seen from Figure 9 that the EPR line is broad and this might be due to dipolar interactions between the manganese ions with mixed valence states [35] . Usually, the antiferromagnetic material has very small EPR intensity. For nanomaterial, the appearance of high EPR intensity is probably due to some of Mn2+ ions located on the surface. When the number of surface atoms increases, the EPR intensity of Mn2+ appears higher [36] .
4. Conclusion
The Mn2O3 nanoparticles were prepared by co-precipitation technique. The sample was characterized by XRD technique revealing the cubic structure of the crystal. The lattice parameter was calculated as 9.4232 Å that confirmed the standard value of Mn2O3 crystal. Using the Debye-Scherer’s formula, D was calculated from the line
![]()
Figure 6. The UV-spectroscopy of Mn2O3 nanoparticles.
![]()
Figure 8. Magnetization (M) as a function of an external applied magnetic field (H) for Mn2O3 nanoparticles.
![]()
Figure 9. EPR spectrum of the Mn2O3 nanoparticles. The inset shows the g-factor of the NPs as a function of the intensity.
![]()
Table 2. The values of Ms, Hc and Mr.
of best fitted and it was found to be 17.3 nm. Using TEM technique, the shape of Mn2O3 NPs was spherical with D = 19.1 nm and it was very close to the one obtained from XRD. PIXE technique had succeeded in determining the Mn content whereas the oxygen content was well determined by RBS technique. As a result, the stoichiometry was determined for the two elements, and both techniques had small errors compared to the nominal values and to each other. RBS and PIXE showed that the sample had very high purity and no contaminations were found. The optical properties of Mn2O3 NPs, using UV-spectroscopy in the range 350 nm - 800 nm, showed that Mn2O3 had very high absorbance in the visible range and that it could be used in solar cells and photo-catalysts. The direct energy band gap was also calculated resulting in 1.24 eV which was very close to the reported values. The FTIR spectrum of the sample was performed in the range 400 - 800 cm−1 showing the existence of the stretching vibration of Mn-O and Mn-O-Mn that indicated the formation of Mn2O3. The magnetic study of the sample showed the antiferromagnetic behavior of the Mn2O3 NPs and a saturation magnetization of 2.642 emu/g. The EPR spectrum showed a high intensity due to the existence of Mn2+ ions on the surface. The g-factor was found to be 1.985 and the magnetic field shift occurred at Bo = 350.5 mT.
Acknowledgements
This research was done in Beirut Arab University (BAU)-Faculty of science Department of physics Beirut- Lebanon in collaboration with Alexandria University-Faculty of science-department of physics-Alexandria- Egypt.
NOTES
*Corresponding author.