Vapor Phase Ammoxidation of 4-Phenyl-o-Xylene into 4-Phenylphthalonitrile on V–Sb–Bi–Zr/γ-Al2O3 Oxide Catalyst ()
1. Introduction
In previous studies [1] [2] ammoxidation reactions of 4-phenyl-o-xylene (I) and 4-phenyl-o-tolunitrile (II) on V?Sb?Bi?Zr/g-Al2O3 oxide catalyst were studied, and mechanism of the products formation, consistent with the observed kinetic regularities, was considered as well.
Creation of aromatic nitriles production on the base of oil hydrocarbons is highly important. Aromatic nitriles, containing various substituents and functional groups, which can be used for the synthesis of thermostable polyimide polymers, insecticides, liquid crystals, phthalocyanine dyes, repellents and other products with high operating abilities, are of great interest among the products of organic synthesis [3] . From these products 4-phe- nylphthalonitrile is an attractive raw material for synthesis of copper tetraphenyltetraazoporphinate (copper tetraphenylphthalocyanine), which, in turn, is used to obtain the phthalocyanine dyes and pigments of turquoise and yellow colors. Usage of 4-phenylphthalonitrile allows substituting high-chlorinated copper phthalocyanine, the preparation of which requires a very complex and environmentally dirty process of copper phthalocyanine chlorination, by chlorine in the melt of aluminum chloride and sodium chloride [4] [5] . As regards the functional-substituted phthalonitriles, it is important to note that due to their application usually as initial compounds for the synthesis of phthalocyanines with a desired structure, and since phthalocyanines, containing alkylamino- or arylamino-groups, are more soluble [6] with the purpose to obtain aminosubstituted dinitriles, a nucleophilic substitution reaction in halogenated phthalonitriles, including 4-bromophthalonitrile, is used [7] .
4-phenylphthalonitrile may be prepared in a single stage by vapor phase ammoxidation, which combines in one process polytypic oxidation reactions and ammonolysis, and is acceptable in economic and technological relation [8] -[10] . Thereupon, according to old-established patent [11] , the preparation of 4-phenylphthalonitrile by the oxidative ammonolysis of 4-phenyl-o-xylene is proposed. Here, on 10%V2O5 - 5.6%CrO3/g-Al2O3 catalyst at temperature 693 K and at molar ratio of 4-phenyl-o-xylene:NH3:O2, equal to 1:39.71:6, 4-phenylphthalo- nitrile is obtained with a yield of 70% and conversion of a substrate in this case is 99%. Study of the vapor phase oxidation and ammoxidation reactions of 3,4-di- and 3,3’,4,4’-tetramethylbiphenyls give an opportunity to obtain the corresponding anhydrides, imides, nitriles of biphenylcarboxylic acids in the yield of 59 - 69 mol.% [12] .
The formation of 4-phenylphthalonitrile by the vapor phase ammoxidation of 4-phenyl-o-xylene with high and average conversion, respectively, in a single process and in a recirculation process is considered in this contribution. It has been established that the base catalyst V-Sb-Bi/g-Al2O3, modified by zirconia oxide, is a good contact for carrying out of this reaction.
Microspheric aluminium oxide, possessing by high mechanical strength, is selected as a carrier. The size of Al2O3 grains allows carrying out the process in a fluidized bed of catalyst. The carrier is previously subjected to a special thermal treatment; samples of the catalysts are prepared by impregnation method of the support by metal salts entering into a composition of the active mass [13] .
Selection of a catalyst and reaction conditions for the selective conversion of 4-phenyl-o-xylene into dinitrile is complicated by the fact that ortho-substituents in the aromatic compounds embarrass the formation of nitriles, but facilitate the formation of imides [8] [14] -[16] . Therefore, the main products of the ammoxidation of o-xy- lene and also 4-bromo- and 4-phenyl-o-xylenes are the corresponding dinitriles and imides of o-phthalic acid, the simultaneous presence of which in the reaction products results in the formation of hard-separable crystal mixture [5] [17] , which adversely affects the efficiency of the whole process [18] .
It is important to note that a possibility of intramolecular interaction on intermediate stages [10] determines the specificity of the kinetic regularities [1] [2] [19] , and selectivity control by means of the regime conditions is complicated by the kinetic features of the reaction. As a result of the carried out studies the following scheme of the formation of the basic products is proposed [1] [20] [21] .
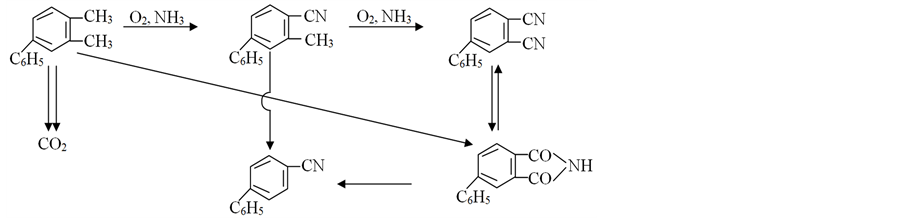
As can be seen from the scheme, ammoxidation of 4-phenyl-o-xylene (I) 4-phenyl-o-tolunitrile (II) is an intermediate product in the formation of 4-phenylphthalonitrile (III), which also provides ammonolysis of 4-phe- nylphthalimide (IV). Formation of 4-phenylphthalimide occurs both as a result of hydrolysis of 4-phenylph- thalonitrile and directly from 4-phenyl-o-xylene. In accordance with the scheme, 4-phenylbenzonitrile (V) is obtained from 4-phenylphthalimide and 4-phenyl-o-tolunitrile.
2. Methods and Apparatus
Kinetic measurements of 4-phenyl-o-xylene conversion and chromatographic separation of catalyzate components, and a quantitative calculation of their content were carried out in accordance with the earlier developed methods [1] , namely according to the method of internal standards and the known formulas. As the standard has been used tetradecane, calibration of 4-phenyl-o-xylene, 4-phenyl-o-tolunitrile, 4-phenylphthalonitrile and 4- phenylpthalimide was carried out relatively the tetradecane. Kinetic measurements were performed in a setup with a vibrating fluidized-bed, gradientless flow reactor of 20 cm3 capacity made from 12Kh18N10T steel. To prevent high-boiling-point products from condensation, part of the setup was thermostated at 500 - 520 K.
The products absorbed by 1,4-dioxane were determined on a Chrome-5 chromatograph equipped with an FID and a column of 1.2 m length. The column packing was Chromaton N-AW (0.2 - 0.25 mm) coated with a mixed stationary phase of Apiezon L (21%) and PEG 40000 (0.5%) or with Polisorb-1 (0.25 - 0.5 mm) alone. Separation of O2 and N2 was carried out on the same chromatograph using a parallel column packed with NaX. Carbon dioxide was determined on LKhM-8MD chromatograph with triethylene glycol butyrate supported on INZ-600 (calcined diatomaceous earth) as the stationary phase.
Analysis of the kinetic data and optimal parameters of the process is made with the use of our software package OptimMe written in C#. Visualization of the results is also carried out in OptimMe. Modular design of the proposed software package provides the opportunity to collect and organize information.
3. Experimental
4-Phenyl-o-tolunitrile (II), 4-phenylphthalonitrile (III), 4-phenylpthalimide (IV), 4-phenylbenzonitrile (V), carbon dioxide, unreacted I, oxygen and the diluent gas nitrogen were determined by gas chromatography in the products of the reaction of 4-phenyl-o-xylene (I) ammoxidation on the V−Sb−Bi−Zr/γ-Al2O3-oxide catalyst. The reaction gases were successively passed through a 1,4-dioxane-filled trap for absorption of nitriles, and III and sulfuric acid-filled trap for absorption of ammonia. The concentration of ammonia at the reactor outlet was determined, by titration of unreacted sulfuric acid from the second trap. The carrier-gas (nitrogen) flow rate was 80 mL/min. The sample injection temperature was 353 K, and the temperature programming rate was 20 K/min.
4. Results and Discussion
For the purpose of finding the optimal conditions of recirculation the results of some regularities of this single process were discussed. The tests were performed in a setup with a vibrating fluidized-bed, gradientless flow reactor in a wide range of parameters variation [1] [2] .
Experimental data, obtained at different molar ratios of 4-phenyl-o-xylene: oxygen in the initial mixture, are presented in Table 1. Increasing the amount of oxygen in the mixture greater than some minimal value (РО2)min leads to a sharp increase in conversion of raw material and selectivity of its conversion into 4-phenylphthaloni- trile, amount of the intermediate 4-phenyl-o-tolunitrile in this case is decreased. The further change in the mole ratios of the initial substances have no essentially influence on the process selectivity (Si) and conversion (α) of 4-phenyl-o-xylene.
As seen from Table 1, upon increasing temperature to 693K 4-phenylbenzonitrile appears in the reaction products. Decrease of oxygen in the mixture below the value (РО2)min leads to reducing in conversion, selectivity of 4-phenylphthalonitrile and CO2, and to increasing the selectivity of the intermediate 4-phenyl-o-tolunitrile. It appears can be explained by the decrease of oxidation degree of a surface of the catalyst, does not resulting, however, in changing the ratio of specific oxygen species, responsible for the reaction course of partial and complete oxidation, which is consistent with data [22] at studying the vapor-phase ammoxidation of toluene and its chlorinated derivatives on the oxide catalysts.
The amount of ammonia in the initial mixture significantly affects the composition of the reaction products. At relatively low mole ratio of NH3:4-phenyl-o-xylene the reaction of deep oxidation of feed stock proceeds predominantly, and the yield of 4-phenylphthalimide is rather high even at very low contact time t (Table 2).
s ![]()
Table 1. Influence of oxygen concentration on parameters of 4-phenyl-o-xylene ammoxidation (contact time t 0.27 s).
![]()
Table 2. Influence of ammonia concentration on parameters of 4-phenyl-o-xylene ammoxidation (t 0.27 s).
With an increase of mole ratio of NH3:4-phenyl-o-xylene, yield of 4-phenylphthalonitrile increases, however, conversion degree of 4-phenyl-o-xylene on V?Sb?Bi?Zr/g-Al2O3-oxide catalyst is not dependent on the partial pressure of ammonia in the reaction mixture. In the presence of large amounts of ammonia the formation of 4- phenylphthalimide is suppressed and the selectivity of 4-phenylbenzonitrile is slightly changes, and above the minimum concentrations (PNH3)min of ammonia, i.e. at PNH3 > (PNH3)min, zero order on ammonia is realized. Reducing the amount of ammonia in the mixture up to the partial pressures PNH3 < (PNH3)min favors to intensification of reactions of deep oxidation of 4-phenyl-o-xylene to CO2 and 4-phenylphthalimide at the expense of reducing the selectivities on mono- and dinitriles. This appears to be connected with the fact that in addition to the main function-nitrogen donor, ammonia also serves as a modifier of the catalyst surface, blocking the centers of deep oxidation of 4-phenyl-o-xylene, reducing the total rate of oxidative conversions of the substrate and forming new catalytic centers. On these centers ammonia competes with O2 [23] and converts labile oxygen- containing intermediates of the incomplete oxidation of raw material into more stable nitrogen-containing products. The high concentrations of NH3, completely displacing O2, suppress intramolecular reactions of closely located side groups among themselves and favors to a course of their isolated conversions into CN-groups [8] [24] [25] .
Dependences of conversion of 4-phenyl-o-xylene and selectivity of the formation of the reaction products on t are presented in Figure 1.
By increasing t selectivity on 4-phenylphthalonitrile increases and selectivity of 4-phenyl-o-tolunitrile formation reduces. However, at high t values 4-phenylphthalimide and 4-phenylbenzonitrile appear in the reaction products, yield of which is increased symbatically to contact time.
Dependences of the yield of the reaction products on temperature are presented in Figure 2. Its increasing up to 693K facilitates growth of conversion selectivity of 4-phenyl-o-xylene into 4-phenylphthalonitrile and reduction of yield 4-phenyl-o-tolunitrile and 4-phenylphthalimide. At high temperatures the reactions of formation of 4-phenylbenzonitrile and CO2 are accelerated, their yield in the range of high temperatures is increased.
On the basis of obtained kinetic regularities the optimal conditions of the single process: 673 K, t = 1.85 - 1.90 s, the mole ratio of I:NH3:air = 1:15:30 are determined.
Selection of the optimal regime was performed on a laboratory setup with a reactor diameter of 40 mm with an organized fluidized bed reactor. Parameters and results of the process are shown below.
![]()
Figure 1. Dependence of the process parameters of 4-phenyl- o-xylene ammoxidation on t at temperature 673 K, the mole ratio of I:NH3:O2 = 1:15:6.3; 1―a; 2―6―selectivity of III, II, CO2, V and IV formation, respectively.
![]()
Figure 2. Dependence of the process parameters of 4-phenyl- o-xylene ammoxidation on temperature at t 1.23 s, the mole ratio of I:NH3:O2 = 1:15:6.3; 1―a; 2―6―selectivity III, II, V, IV and CO2 formation, respectively.
As seen from the presented data, 4-phenylphthalonitrile with yield of 83.10 mol.% counting on the taken 4- phenyl-o-xylene can be obtained on V-Sb-Bi-Zr/g-Al2O3 oxide catalyst. Since the separation of 4-phe- nylphthalonitrile from impurities of 4-phenyl-o-tolunitrile, 4-phenylbenzonitrile and 4-phenylphthalimide, especially from crystal 4-phenylphthalimide, is essentially impeded, and the requirements to 4-phenylphthalonitrile as an initial substance for phthalocyanine pigments formation are extremely rigid [19] , the technological scheme, which provides an average conversion of 4-phenyl-o-xylene with recirculation would be more appropriate.
Analysis of the kinetic data (Table 2) shows, that formation of 4-phenylphthalimide occurs at high concentration of ammonia (starting with 15 moles of NH3 to 1 mole of the substrate) by hydrolysis of 4-phenylphthaloni- trile with water (see scheme), which is the product of concurrent reactions [5] [17] of ammoxidation-oxidative dehydrogenation and deep oxidation of 4-phenyl-o-xylene. In the range of average conversion of 4-phenyl-o- xylene at low contact time because of the low concentration of reactive H2O the aimed dinitrile has no time to be subjected to the secondary conversion with imide formation.
Under these conditions the results of ammoxidation of a mixture of 4-phenyl-o-xylene and 4-phenyl-o-tolu- nitrile are presented in Table 3. As seen, a practically quantitative yield of 4-phenylphthalonitrile counting on the unreacted 4-phenyl-o-xylene can be provided. As distinct from a single process, reaction running on the indicated catalyst at t 0.27s, 653 - 673 K and a mole ratio of I:air:NH3 = 1:30:15 with 39.9% - 55.8 % conversion and recirculation of unreacted 4-phenyl-o-xylene and the intermediate 4-phenyl-o-tolunitrile increases the selectivity to 96.14% - 97.72% on 4-phenylphthalonitrile and reduces the quantity of forming by-products (4-phe- nylph- thalimide, 4-phenylbenzonitrile) and fraction of deep oxidation to CO2.
Herewith, a recirculation coefficient (ratio of the total reactor loading to fresh feed) is ~1.79 - 2.51. Therefore, recirculation gives a possibility to exclude the technological purification stage of the aimed 4-phenylphthaloni- trile and makes the process continuous, highly selective and productive. In spite of the fact that, the process parameters at 633 K are high too, however, the decrease of temperature from the optimum 653 - 673 K due to decreasing of the conversion (Table 3) leads to growth of the recirculation coefficient up to 4. It can lead to increase of costs, connected with increased expenses for recirculate pumping (returned into the process unreacted 4-phenyl-o-xylene and formed 4-phenyl-o-tolunitrile, i.e. it is necessary to find such a conversion at which the costs on the recirculation would not exceed the profit associated with the growth of productivity.
If to consider, that the decrease of the depth of conversion for a single process leads to reduction of the reaction volume, and, hence, to an increase of productivity of the reactor, then at the recirculation the increase of selectivity on 4-phenylphthalonitrile causes a highly productive process. At recirculation catalyst productivity is
![]()
Table 3. Ammoxidation of 4-phenyl-o-xylene and 4-phenyl-o-tolunitrile mixture (t 0.27 s, mole ratio (I + II):O2:NH3 = 1:6.3:15).
~3 times higher than in the single process that at high conversion of 4-phenyl-o-xylene is 176.4 g/l of catalyst per hour [5] . Furthermore, in the single process because of the high degree of conversion of 4-phenyl-o-xylene yield of CO2, the product of a highly exothermic reaction of the complete oxidation increases, which, in turn, complicates the effective removal of heat of the reaction and control by the reactor [5] . Since a problem of selectivity is one of the central problems in the theory of catalysis, selectivity of the catalyst operation is of fundamental importance, i.e. effective contact from the plurality of thermodynamically possible oxidative conversions of a substrate accelerates preferably one reaction.
At recirculation 4-phenylphthalonitrile and CO2 are the only reaction products. Contact gases include O2, NH3, N2, CO2, 4-phenyl-o-xylene, 4-phenyl-o-tolunitrile and 4-phenylphthalonitrile. The unreacted 4-phenyl-o-xylene and forming as the intermediate 4-phenyl-o-tolunitrile after separation from 4-phenylphthalonitrile are returned into the reactor. Therefore, at the technological design of the process separating and trapping of the reaction products, present in the contact gas, isolating of the 4-phenylphthalonitrile from the products mixture, returning of the unreacted 4-phenyl-o-xylene and 4-phenyl-o-tolunitrile into the reactor, regeneration of excess ammonia and its recirculation into the reactor should be provided.
Quality parameters of 4-phenylphthalonitrile obtained at recirculation of 4-phenyl-o-xylene are shown below.
Content of the base substance, mass. % 99.60 - 99.78
Melting point, K 436.0 - 437.0
Residue on ignition, mass. % 0.04 - 0.05
Humidity, mass. % 0.18 - 0.35
Impurity of 4-phenylphthalimide is absent
5. Conclusion
Thus, the proposed method (recirculation) gives a possibility to create the effective single-stage technological process of 4-phenylphthalonitrile formation by ammoxidation reaction of 4-phenyl-o-xylene. Results of the research confirm the advantage of the developed software package OptimMe comparing to other softwares that found its application in the field of chemistry and petrochemistry..
Acknowledgements
Used in this work software package OptimMe was developed by the support of Science Foundation of “SOCAR” under the grant project ET-27 (15/10/2014) at the Institute of Catalysis and Inorganic Chemistry named after Acad. M. F. Nagiyev.
NOTES
*Corresponding author.