Valence Stabilization of Polyvalent Uranium Ions in Presence of Some Organic Additives during Extended Gamma Irradiation of Their Aqueous Acidic Solutions ()
1. Introduction
In modern radiochemical practice, radiation chemical reactions in water and aqueous systems are highly important, particularly in applied nuclear technology.
Gamma irradiation of aqueous solutions normally involves formation of primary water radiolysis products which can effectively interact with the existing chemical species in the irradiated systems. This probably leads to changes in the valence state of any existing polyvalent ions and therefore can seriously interfere with the mechanism of any ongoing chemical separation process. Therefore, valence stabilization of polyvalent metallic ions in aqueous acidic solutions when present in a strong gamma irradiation field is of particular interest. For example, stabilization of actinide ions, particularly of U, Pu and Np in a certain valence state while reprocessing of spent nuclear reactor fuel rods may be very useful in the various steps involved in the process [1] .
The nature of the processes that leads to valence stabilization of polyvalent ions during extended gamma irradiation has been reported before on using simple iron ions. Thus, valence stabilization of Fe(II) ions in aqueous acidic solutions during extended gamma irradiation has been studied using inorganic additives [2] as well as some organic additives [3] [4] to the irradiated solutions. It has been found that valence stabilization of Fe(II) ions depends on the competition reactions occurring between the primary water radiolysis products and the polyvalent ions or the organic additive present in the irradiated solution. The chemical structure of the organic additive and the amount used determines the extent of valence protection of the polyvalent ions.
In the present work, valence stabilization of uranium ions during extended gamma irradiation of its aqueous acidic solutions, containing different organic additives, has been studied in detail. Uranium ions are frequently encountered in numerous separations and determinations in applied radiochemical practice.
2. Experimental
In the present work, aqueous acidic solutions of polyvalent uranium ions in presence of different classes of organic compounds, used as additives e.g. organic acids, aldehydes, alcohols, phenol, 4-aminopyridine and hydrazine hydrate, have been subjected to extended gamma irradiation aiming at studying the protective effect of these additives on the existing uranium ions.
2.1. Materials
Uranyl sulfate (UO2SO4∙3.5H2O; M.W. 429.19) and A.R.Arsenazo-1 Trisodium Salt (M.W. 614.28), Sulfuric acid (98%), Sp.gr. 1.84 and Hydrochloric acid (35% - 37%) Sp.gr. 1.18 were obtained from B.D.H. Co., GB.
Reagent grade Methanol, Ethanol and n-Propanol were obtained from Cambarian Chemicals, GB. Acetaldehyde and Propionaldeyde and also chemically pure Formic acid, Sp.gr. 1.231(100%) were supplied from Prolabo Co., France, Acetic acid for analysis, 99% - 100%, Sp.gr. 1.055 was obtained from Fein Chemie K-H, Kallies KG, Germany. Propionic acid was obtained from May & Baker Co., GB. Phenol, M.W. 94.11, Sp.gr.1.07 B.P. 41˚C, 4-Aminopyridine, M.W. 94.12, M.P. 158˚C and Hydrazine hydrate, M.W. 50.06, Sp.gr. 1.03, B.P. 119˚C were obtained from May & Baker Co. GB.
All chemicals except alcohols were used without further purification. All alcohols were distilled twice over freshly ignited and cooled Calcium oxide.
2.2. Preparation of Stock Solutions
All solutions were prepared using double distilled water which was previously boiled, cooled and kept in air tight glass flasks.
2.2.1. Preparation of U(VI) Solution
4.29 grams of A.R. Uranyl Sulphate (UO2SO4∙3.5H2O) necessary to prepare one liter of 10−2 M 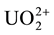 solution were dissolved in about 100 ml of double distilled water, transferred quantitatively into a one liter volumetric flask together with 400 ml. water containing 22.2 ml conc.
solution were dissolved in about 100 ml of double distilled water, transferred quantitatively into a one liter volumetric flask together with 400 ml. water containing 22.2 ml conc. 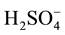 and the solution was completed to the mark. The uranium content was then determined gravimetrically by precipitation with ammonia and after separation of the formed precipitate it was ignited to constant weight and finally weighed, as U3O8 [5] . The concentration of U(VI) was exactly about 10−2 M in 0.8 N H2SO4.
and the solution was completed to the mark. The uranium content was then determined gravimetrically by precipitation with ammonia and after separation of the formed precipitate it was ignited to constant weight and finally weighed, as U3O8 [5] . The concentration of U(VI) was exactly about 10−2 M in 0.8 N H2SO4.
2.2.2. Preparation of U(IV) Solution
U(IV) solutions were prepared by reducing hexavalent uranium in acidic solutions by amalgamated A.R. Zinc packed in a reduction column (Jones reductor). Thus, 50 ml of 10−2 M uranyl sulfate solution (0.8 N H2SO4) to which was added one ml conc. H2SO4 and about 50 ml water were passed through the reduction column at a rate of 3ml/min and the solution was quantitatively collected together with the column washings, 15 ml portions, for three times. A stream of air was passed through the resultant solution for 10 minutes to oxidize any U(III) formed to U(IV) whereby the dark green solution changed to bright apple green color, characteristic of U(IV) [6] . The resultant solution was quantitatively transferred and completed to the mark in 250 ml volumetric flask. The exact concentration of the formed U(IV) was determined titrimetrically using standard 0.1 N Ceric sulfate solution at 50˚C using ferroin indicator [7] . The concentration of U in the solution was 2 × 10−3 M and 0.16 N in H2SO4. Freshly prepared U(IV) solutions were always used in the irradiation experiments.
2.2.3. Preparation of the Organic Additives Solutions
Stock solutions of organic additives (0.16 M) of Methanol, Ethanol, Propanol, Acetaldehyde, Propionaldehyde, Formic and Acetic acids were prepared by introducing the appropriate amount of the pure compound into 500 ml volumetric flasks and completing to the mark with double distilled water. Exactly about 0.16 M solutions of Phenol, 4-Aminopyridine and Hydrazine hydrate were prepared as described before [4] .
2.3. Preparation of Uranium Ions/Organic Additive Solutions to Be Subjected to Gamma Radiations
2.3.1. U(VI) Solutions
Aliquots of 5 ml of 10−2 M 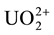 solution were quantitatively transferred into 50 ml volumetric flasks together with certain amounts of the organic additives and the solutions were completed to the mark after adding the necessary amounts of H2SO4 to give a final concentration of 0.08 N H2SO4. The resultant solutions were then introduced into the glass irradiation tubes and irradiated.
solution were quantitatively transferred into 50 ml volumetric flasks together with certain amounts of the organic additives and the solutions were completed to the mark after adding the necessary amounts of H2SO4 to give a final concentration of 0.08 N H2SO4. The resultant solutions were then introduced into the glass irradiation tubes and irradiated.
2.3.2. U(IV) Solutions
50 ml aliquots of 2 × 10−3 M U(IV) solution (0.16 N H2SO4), prepared as described before were quantitatively introduced into 100 ml volumetric flasks together with the necessary amount of different organic compounds and the resultant solutions were completed to the mark after adding the necessary amounts of H2SO4 to give a final concentration of 10−3 M U(IV) in 0.08 N H2SO4, The prepared solutions were then introduced to the glass irradiation tubes and irradiated.
2.4. Irradiation of Sample Solutions
Glass irradiation tubes; 15 cm long and 2.5 cm in diameter were used; each was provided with a neck 1 cm in diameter ending with a ground glass stopper.
Gamma Irradiation was carried out using a Canadian 60Co gamma cell-220, in the Nuclear Physics Department, Atomic Energy Authority, Egypt, for extended periods of time at absorbed dose rate 360 Gy/hour. The irradiation dose of the gamma cell was occasionally checked by the ferrous sulphate method.
2.5. Analysis of the Irradiated Solutions
The concentration of existing (remaining) U(IV) in the continuously irradiated solutions was followed by spectrophotometric analysis. Thus, one ml samples were taken from the irradiated solution at intervals and after adjusting the pH at 2 by very dilute H2SO4, one ml Arsenazo 1 was added (75 mg/l00 ml water). The solution was completed to the mark in a 10 ml volumetric flask and the absorbance was measured at 580 nm within three minutes. The concentration of U ions was obtained by using a a preconstructed calibration curve: U concentration- absorbance. The molar absorbtivity was found to be 18,500 dm3∙mol−1∙cm−1. Measurements of existing U(IV) concentrations in the irradiated solutions, were carried out twice daily. By comparing the absorbance of the irradiated sample with that of an unirradiated sample determined under the same conditions i.e. at pH 2 and 0.08 N H2SO4, the percent existing U(IV) in the continuously irradiated solutions was determined.
Spectrophotometric measurements were carried out using a Shimadzu UV-Vis double beam spectrophotometer type UV-210A. A Radiometer type PO3 pH meter with a calomel, glass or Pt electrodes was used for pH measurements or potentiometric titrations
2.6. Reaction Rate Constants
In the present work, the reaction rate constants, k values are expressed in dm3∙mol−1∙s−1 and are used as given in Anbar [8] [9] . For simplicity the rate constant values are used without dimensions.
3. Results and Discussions
It is well known that uranium has two rather stable oxidation states U(IV) and U(VI). In air U(IV) is relatively unstable and undergoes autoxidation to U(V) by atmospheric air which further disproportionates to U(IV) and U(VI). The decay of U(IV) in air with time in acidic solutions at room temperature was followed spectrophotometrically. It was found that a 10−3 M U(IV) solution was almost completely oxidized in about 120 hours, as shown in Figure 1(a). On the other hand, upon gamma irradiation of a 10−3 M U(IV) acidic solution at absorbed dose rate 360 grays per hour, uranium ions decayed completely after receiving a total dose of about 2 kGys after about 5.55 h of irradiation, as shown in Figure 1(b). Under the same conditions U(VI) remained unaffected by gamma irradiation.
It is well known that gamma irradiation of aqueous uranium solutions leads to the generation of both reducing
(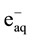 ,
,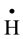 ) and oxidizing (
) and oxidizing (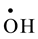 , H2O2) species which readily interact with the solutes present [10] . Hexavalent uranium has been reported to react with
, H2O2) species which readily interact with the solutes present [10] . Hexavalent uranium has been reported to react with 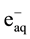 and
and 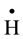 with a rate constant 7.4 × 1010 and 4.5 × 107 dm3∙mol−1∙s−l respectively whereas U(IV) is expected to be oxidized by
with a rate constant 7.4 × 1010 and 4.5 × 107 dm3∙mol−1∙s−l respectively whereas U(IV) is expected to be oxidized by 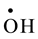 and H2O2 [11] . No observable reduction of
and H2O2 [11] . No observable reduction of
U(VI) to U(IV) were recorded when the solutions were irradiated [10] .
The oxidation of U(IV) upon gamma irradiation in air has been assumed to occur in the following sequence [12] .
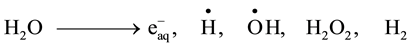
 which are abundantly formed in water radiolysis are readily converted to
which are abundantly formed in water radiolysis are readily converted to 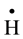 particularly in acid solutions [13] .
particularly in acid solutions [13] .
In presence of oxygen
![]()
Figure 1. (a) Decay line of 10−3 M U(IV) soluation during storage; (b) Radiolytic decay line of U(IV) solutions upon gamma irradiation (Dose rate 360 Gy/h).


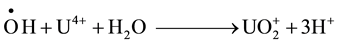
![]()
![]()
![]()
This mechanism indicates the important role played by ![]() radicals and H2O2. Nevertheless, it has been
radicals and H2O2. Nevertheless, it has been
reported that H2O2 does not react with U(VI) [14] and reacts only very slowly with U(V) and U(IV) [11] . Therefore, it is possible to conclude that in a U(IV) acidic solution particularly in a very limited supply of oxy
gen, ![]() and
and ![]() are the main players in the occurring oxidation process.
are the main players in the occurring oxidation process.
In the present work the protective effect of some organic compounds e.g. aliphatic acids, aldehydes, alcohols and also phenol, 4-aminopyridine and Hydrazine hydrate against radiolytic oxidation of U(IV) ions in acidic solutions will be discussed in detail.
3.1. Valence Stabilization of U Ions in Acidic Aqueous Solutions during Gamma Irradiation in Presence of Some Organic Alcohols
Aqueous acidic U(IV) solutions were continuously subjected to gamma radiations in presence of increasing amounts of each of methanol, ethanol or propanol. The % existing U(IV) in the irradiated solutions was followed spectrophotometrically twice daily during continued gamma irradiation. The results obtained are shown in Figure 2.
It could be observed from these data that the use of increasing quantities of the organic additives, methanol, ethanol or propanol with 10−3 M U(IV) induced variable protection during extended gamma irradiation of the solutions. At the beginning of irradiation around 50% of the exiting U(IV) were oxidized and then were gradually reduced until a maximum value of about 70% U(IV). This is followed by a gradual oxidation of U(IV) until all Uranium ions were oxidized after the complete consumption of the organic additive. The area under the solid lines in Figure 2 was taken as a measure of the actual protection exerted by the added organic alcohol on U(IV). It is clear that methanol has got the greater capacity in protecting U(IV) as compared to ethanol or propanol. Propanol has the least capacity.
For better understanding of the prevailing reactions we used U(VI) instead of U(IV) in the irradiated solution. It is well known that hexavalent uranium in aqueous acidic solutions is not affected by gamma irradiation. However, in presence of organic alcohols in the irradiated U(VI) acidic solutions, gradual reduction occurred depending on the alcohol used and its concentration. It has been reported that reduction of U(VI) very probably occurs by the following reaction [10]
![]()
![]()
At the same time ![]() radicals will be principally engaged in scavenging reactions with the alcohol mole
radicals will be principally engaged in scavenging reactions with the alcohol mole
cules present.
The data presented graphically in Figure 3 shows that at the beginning of irradiation U(VI) starts a gradual reduction process until about 70% of U(VI) has been reduced to U(IV). Then U (IV) concentration remained almost stable until the organic additive was significantly consumed whereby U (IV) tended to be gradually oxidized until it was finally transformed to U (VI). Again, methanol and ethanol have the greatest capacity of protection while propanol has got the least capacity.
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 2. Percent existing U(IV) in g-irradiated 10−3 M U(IV) solution (0.08 N H2SO4) containing Methanol (a); Ethanol (b) or Propanol (c) at concentrations: 1: 8.0 × 10−3 M, 2: 16.0 × 10−3 M, 3: 32.0 × 10−3 M (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 3. Percent existing U(IV) in g-irradiated 10−3 M U(VI) solutions (0.08 N H2SO4) containing Methanol (a); Ethanol (b) or Propanol (c) at concentrations: 8.0 × 10−3 M (1) 16.0 × 10−3 M (2) 32.0 × 10−3 M (3) (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
3.2. Valence Stabilization of U(IV) Ions in Acidic Aqueous Solutions during Gamma Irradiation in Presence of Some Organic Aldehydes
Aqueous acidic uranium solutions containing various concentrations of acetaldehyde and propionaldehyde were continuously irradiated and the change of the uranium ions concentration was followed spectrophotometrically. The results are shown in Figure 4(a) and Figure 4(b).
From these results it is clear that in case of acetaldehyde the U(IV) ions remained in the tetravalent state until the organic species was almost consumed whereby the U(IV) concentration gradually decreased until it was totally oxidized to the hexavalent state.
In presence of Propionaldeyde, no features of protection of U(IV) were observed and irrespective of the amount of the used alcohol, U(IV) gradually decayed over a smaller range of absorbed doses at a rather faster rate than in case acetaldehyde.
The behavior of U(VI) solutions during gamma irradiation in the presence of acetaldehyde is shown in Figure 5.
![]()
![]() (a) (b)
(a) (b)
Figure 4. Percent existing U(IV) in g-irradiated 10−3 M U(IV) solutions (0.08 N H2SO4) containing: (a) Acetaldehyde: 8.0 × 10−3 M (1), 16.0 × 10−3 M (2), 32.0 × 10−3 M (3) (b) Propinaldehyde: 8.0 × 10−3 M (1), 16.0 × 10−3 M (2), 32.0 × 10−3 M (3) (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
![]()
Figure 5. Percent existing U(IV) in g-irradiated 10−3 M U(VI) solutions (0.08 N H2SO4) containing various concentrations of Acetaldehyde as follows: (1) 8.0 × 10−3 M (2) 16.0 × 10−3 M (3) 32.0 × 10−3 M (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
It is clear that at first complete oxidation of U(IV) takes place. This is followed by a gradual reduction of U(VI) to U(IV) until a certain maximum value was reached. This is followed by a gradual decay of the formed U(IV) until it is completely exhausted.
3.3. Valence Stabilization of U(IV) Ions in Acidic Aqueous Solutions during Gamma Irradiation in the Presence of Some Organic Acids
The change of U(IV) concentration during gamma irradiation is shown in Figure 6. It could be observed that formic acid can effectively protect quadrivalent uranium ions. At the same time acetic and propionic acids did not stop the gradual decay of U(IV) upon continued irradiation even on using 30 times excess of the additive.
In case of using hexavalent uranium in the irradiated solution instead of U(IV) the results are shown in Figure 7. Only formic acid was capable of exerting protection on the tetravalent uranium formed by gradual reduction of the initially used U(VI).
3.4. Valence Stabilization of U(IV)Ions in Acidic Aqueous Solutions during Gamma Irradiation in Presence of Phenol, 4-Aminopyridine and Hydrazine Hydrate
Solutions containing 10−3 N U(IV) ions and increasing amounts of phenol, 4-ethylpyridine and hydrazine hydrate, in the concentration range 8 - 32 mmol were continuously subjected to gamma irradiation. Results of the analysis of the irradiated solutions to determine the concentration of U(IV) are shown in Figure 8. It could be observed that in case of phenol no protective effect was noticed within the concentration range of phenol used. On the other hand on using 4-aminopyridine rapid decay of U(IV) occurred while on using hydrazine hydrate more rapid decay of U(IV) occurred irrespective of the amount of the organic additive used. It is interesting to note that on studying the protective effect of these additives in case of irradiated Fe(II) solutions, previously reported [4] , it was found that these compounds have a clear and good protective effect on divalent iron ions during extended gamma irradiation. It seems that U(IV) ions, being stronger reducing agent as compared to Fe(II) ions is much more capable of directly interacting with the oxidizing species in the irradiated aqueous acidic solutions containing U(IV) thus leading to a reduced protective effect.
3.5. Effect of Concentration of the Organic Additives on Valance Protection of Uranium Ions during Extended Gamma Irradiation of Their Aqueous Solutions
In order to define the relationship between the amount of the organic additive used and the resultant protection
![]()
Figure 6. Percent existing U(IV) in g-irradiated 10−3 M U(IV) solutions (0.08 N H2SO4) containing various concentrations different organic acides as follows: −1, 2, 3: 8.0, 16.0 and 32 mM formic acide 4: 8.0, 16.0 and 32 mM actic acide 5: 8.0, 16.0 and 32 mM propionic acide (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
![]()
Figure 7. Percent existing U(IV) in g-irradiated 10−3 M U(VI) solutions (0.08 N H2SO4) containing various concentrations of formic acid as follows: 1: 8.0 × 10−3 M, 2: 16.0 × 10−3 M, 3: 32.0 × 10−3 M (ــــــــــــــ, actual protection line; - - - - -, 100% protection line).
![]()
Figure 8. Percent existing U(IV) in g-irradiated 10−3 M U(VI) solutions (0.08 N H2SO4) containing various concentrations of: A―Hydrazine hydrate B―4-Aminopyridine C―Phenol [o―8 m mol, 16 m mol, ・32 m mol, D―1.6 m mol, 3.2 m mol, 6.4 m mol.
of U(IV) in gamma irradiated systems the areas under the actual protection lines in Figures 2-7, were determined in cm2 and were taken as a measure of the occurring protection. These values were plotted against the corresponding concentrations of the organic additives used.
The results obtained are shown in Figure 9. Good linear relationships could be observed between the amount of the organic additive used and the resultant area under the protection lines in cm2. This confirms the dependence of the extent of occurring valence protection of U(IV) ions on the amount of organic additive used.
In order to compare the protective capacity of different organic additives occurring in irradiated samples containing different organic additives, the percent total protection values were determined in each case as follows:
![]()
where Ap is the area under the protection line and At is the total area under the corresponding 100% protection
![]()
![]() (a) (b)
(a) (b)
Figure 9. The relationship between the organic additive concentration and the area under the protection curve (cm2) for different U ions solutions: (a) 10−3 N U(IV) solutions containing 1―Formic acid, 2―Methanol, 3―Acetaldehyde, 4―Propanol, 5― Ethanol; (b) 10−3 N U(VI) solution containing 1―Formic acid, 2―Acetaldehyde, 3―Ethanol, 4―Methanol, 5―Propanol.
![]()
Table 1. Percent overall protection during extended gamma irradiation of Uranium ions (10−3 M) acidic solutions, in presence of various concentration of some organic additives.
A―Concentration used, mM, B―Total absorbed dose, kGy; C―Per cent taken as a measure of the extent of total overall protection.
line.
The % of total protection was taken as a measure of the occurring protection as a result of the presence of a given amount of organic additive. The results obtained are given in Table 1, and are represented graphically in Figure 10 for U(IV) solutions. From these results it could be observed that different additives have different protection capacity. Formic acid and methanol have the highest capacity of valence protection (78% - 88%) during irradiation while propanol has the least capacity.
4. Conclusions
1) The average percent protection of U(IV) ions in acidic solutions when subjected to extended gamma irradiation amounted to 79% and 81% respectively in presence of methanol and formic acid, 70% and 68.5% respectively in presence of acetaldehyde and ethanol. Propanol addition has the least protective effect amounting to 54%. These protection levels prevail until the organic additive is consumed.
![]()
Figure 10. Percent protection of U(IV) in g-irradiated 10−3 M of U(IV) solutions (0.08 N H2SO4) containing various concentrations of: 1―Formic acid, 2―Acetaldehyde, 3―Metha- nol, 4―Ethanol, 5―Propanol.
2) On using U(VI) solutions in presence of different organic additives reduction occurs gradually and the formed U(IV) was found to have the following average percent protection: methanol and formic acid 63% and 69% respectively while in case of using ethanol, propanol and acetaldehyde the percent protection amounted to 50%, 24% and 35% respectively. These protection levels exist until the complete exhaustion of the organic additive used.
3) 4-ethylpyridine, phenol and hydrazine hydrate which were reported before [4] to be highly effective in protecting Fe(II) ions during extended gamma irradiation, were found to be very slightly effective in protecting U(IV) solutions due to the fact that U(IV) is a more powerful reducing agent than Fe(II) and consequently more capable of interacting with the oxidizing species resulting from water radiolysis.
NOTES
*Corresponding author.