Inclusion-Interaction Assembly Strategy for Constructing pH/Redox Responsive Micelles for Controlled Release of 6-Mercaptopurine ()
1. Introduction
β-Cyclodextrin (β-CD) is a natural cyclic oligosaccharide composed of 7 glucose units linked by α-1, 4 glycosidic bonds, with a hydrophilic outer surface and a hydrophobic inner cavity [1] - [3] . Due to inclusion interactions, a wide range of hydrophobic drugs can be embedded into the hydrophobic cavities to form inclusion complexes, thus significantly increasing the solubility and permeability of the drugs [4] . Moreover, grafting β-CD onto polymers can result in materials with unique features and excellent biocompatibility [5] . A lot of β-CD containing polymers have been prepared and utilized to build supramolecular systems [6] .
In recent years, our group has prepared a variety of polymeric micelles, in which cores and shells are linked by covalent bonds [7] - [10] . Recently, we developed a novel hydrogen-bonding strategy to build pH-sensitive micelles, using hydrophilic polymer and hydrophobic drug as building blocks [11] . It resulted in a micelle, in which only hydrogen bonds rather than covalent bonds existed between core and shell. Further, it achieved the integration of drug-loading and self-assembly in preparing drug-loaded micelles, avoiding the multiple steps used in the covalent-bond method. This result aroused our interest to construct a novel non-covalently connected micelle, with inclusion interaction serving as driving force.
We assumed that, if a hydrophilic β-CD containing polymer and a dimer of hydrophobic drug could form an amphiphilic inclusion complex, with the dimer being partially embedded into the cavity of β-CD moiety, the complex should be able to self-assemble into a micelle, with dimer as core and the polymer as shell. Meanwhile, it was mentioned that the GSH concentration in human cell (millimolar level) was much higher than that in bloodstream (micromolar level), and that the GSH concentration in tumor cells was at least 4-fold higher than that in normal cells [12] . Therefore, if we chose the dimer that two monomer units were joined by a disulfide bond, it would be able to remain stable in bloodstream, and become easy to cleavage once the micelle had entered into tumor cells, and the controlled release of drug might be achieved.
Based on the above assumptions, dimer of 6-mercaptopurine (DMP) was determined. First, from the chemical structure, DMP was a homodimer consisting of two 6-mercaptopurine (6-MP) monomers joined by a disulfide bond. Second, DMP had good hydrophobility and could form an inclusion complex with β-CD with its molecule being partially embedded into the cavity of β-CD. Third, 6-MP was an anti-cancer drug with low bioavailability (about 16%) as well as short plasma half-time (0.5 - 1.5 h), due to its poor solubility and a free sulfhydryl group easily reacting with the plasma proteins [13] . With the micellization of DMP, these two defects could be avoided.
Carboxymethyl chitosan (CMCS), obtained from carboxymethylation of chitosan, was also determined. On one hand, CMCS was biopolymer with good aqueous solubility, low toxicity and good biocompatibility [14] . On the other hand, it was reported that polymeric micelles with CMCS as shells usually could swell in slightly acid aqueous solution, due to a lot of reversibly ionized groups existing in their structures [10] . Taking account of the slightly acid intracellular environments of tumor cells (lysosome pH 4.5 - 5.0 and endosome pH 5.0 - 6.5) [15] , this result might contribute to exposing the disulfide bonds in the core of the micelle to the GSH in tumor cells.
The aim of this study was to employ an inclusion-interaction assembly strategy to construct a novel pH/redox responsive micelle system for controlled release of 6-MP. At first, a hydrophilic β-CD grafted carboxymethyl chitosan (CMCS-g-β-CD) and a dimer of 6-MP were synthesized. Then, an amphiphilic inclusion complex (CMCS-g-β-CD・DMP) was prepared with the dimer being partially embedded into the cavity of β-CD moiety. It self-assembled into micelles in distilled water. Their structure and morphology were observed by transmission electron microscopy (TEM). The stability, pH-sensitivity and reduction-response were investigated by dynamic light scattering (DLS). Their stimuli-responsive release properties and anti-tumor activity were also studied.
2. Materials and Methods
2.1. Materials
Chitosan (CS, MW: 560 kDa, degree of decetylation: 91%) was purchased from Yuhuan Ocean Biochemical Co., Ltd. (
Zhejiang
,
China
). N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide (EDC), β-Cyclodextrin (β-CD), N-Hydroxysuccinimide (NHS) and 6-Mercaptopurine (6-MP) were all purchased from Aladdin Reagent Inc. (Shanghai, China). All other chemical reagents were of analytical grade and used without further purification. HeLa cells were purchased from American Type Culture Collection (ATCC) and grown in Dulbecco’s Modified Eagel medium (DMEM) containing 10% FBS.
2.2. Synthesis of CMCS
Chitosan (
10.0 g
) was stirred with 100 mL isopropanol at room temperature for 24 h. This solution was then filtered and the filter residue was added with 40 mL NaOH aqueous solution (50 wt%). The mixture was stirred for 1 h and placed into a refrigerator at −20˚C for 24 h. After the frozen mixture was thawed, chloroacetic acid (
20.0 g
) dissolved in 20 mL isopropanol was dropwise added. This solution was stirred overnight below 10˚C and then filtered. The filter residue was dialyzed (MWCO 14,000) against distilled water and lyophilized to obtain CMCS. The degree of substitution (DS) of carboxymethyl groups was determined as ~34% by 1H-NMR.
2.3. Synthesis of CM β-CD
β-CD (
5.68 g
) was stirred with 40 mL NaOH aqueous solution (25 wt%) in a three-necked flask at 65˚C for 1 h. Then, chloroacetic acid (
0.473 g
) dissolved in 10mL distilled water was dropwise added into the flask with stirring. The mixture was stirred at 65˚C for 8 h. Then, the reaction mixture was neutralized and precipitated with excess amount of methyl alcohol. After the precipitate was collected and dried, CM β-CD was obtained. The DS of carboxymethyl groups was determined as ~76% by 1H-NMR.
2.4. Synthesis of DMP
DMP was synthesized following the method mentioned in the literature [16] . 6-Mercaptopurine (
0.500 g
) was dissolved in 300 mL pH 7.6 phosphate buffer solution (PBS) at 60˚C. After the solution was cooling down,
0.406 g
I2 dissolved in 2 mL DMSO was dropwise added. This solution was stirred for 2 h at room temperature. Then, the light yellow precipitate was collected by filtration and washed by distilled water (4 × 100 mL). The precipitate was then dried and DMP was obtained.
2.5. Synthesis of CMCS-g-β-CD
CM β-CD (
2.39 g
), EDC (
0.384 g
) and NHS (
0.230 g
) were dissolved in 60 mL distilled water and stirred for 1 h at room temperature. Then, CMCS (
0.328 g
) dissolved in 15 mL distilled water was dropwise added into the solution where the pH value was maintained at 7. The solution was stirred for another 6 h and then dialyzed (MWCO 14,000) against distilled water for 72 h. The dialysate was lyophilized and CMCS-g-β-CD was obtained. The DS was determined as ~59% by 1H-NMR.
2.6. Preparation of CMCS-g-β-CD・DMP
CMCS-g-β-CD (
1.39 g
) was dissolved in 100 mL PBS (pH 7.4) at 60˚C. DMP of various amounts (
0.151 g
,
0.302 g
and
0.453 g
) were dissolved in 2 mL DMSO and then respectively added into the solutions with stirring. The reaction mixtures were stirred for 6 h and then dialyzed (MWCO 14,000) against distilled water for 8 h. After the dialysates were freeze-dried, CMCS-g-β-CD・DMP with various DMP contents were obtained. Their drug contents were determined by elementary analysis, as summarized in Table 1.
2.7. Characterization
The FT-IR spectra of CS, CMCS, β-CD, CM β-CD, DMP, CMCS-g-β-CD, the physical mixture of CMCS- g-β-CD and DMP and CMCS-g-β-CD・DMP were determined by a FT-IR spectrometer (Avator 360, Nicolet, MA, USA) using KBr pellets. The 1H-NMR spectra of CMCS, CMCS-g-β-CD, CMCS-g-β-CD・DMP were determined on a Varian 600 spectrometer (Varian,
USA
) at 600 MHz using D2O as solvents. The DSC profiles of the physical mixture of CMCS-g-β-CD and DMP, CMCS-g-β-CD・DMP and CMCS-g-β-CD were determined by a DSC calorimeter (
200F
3, NETZSCH, German) with a temperature range of 60˚C - 180˚C.
2.8. Fabrication and Characterization of CMCS-g-β-CD・DMP Micelles
CMCS-g-β-CD・DMP (
0.01 g
) was dispersed in 10 mL PBS (pH 7.4) and stirred at room temperature for 0.5 h.
![]()
Table 1. Characteristic data of CMCS-g-β-CD・DMP with different DMP contents.
Then, this dispersion was sonicated by a probe type sonifer (JY92-2D, made by Ningbo Xinzhi Bio-tech Co., Ltd) at 60 W for 3 min and a CMCS-g-β-CD・DMP micelle solution was obtained. The micelles prepared from CMCS-g-β-CD・DMP1, CMCS-g-β-CD・DMP2 and CMCS-g-β-CD・DMP3 were named M-1, M-2 and M-3, respectively. Their mean diameters and distributions were determined by DLS using a Nano-ZS3600 (
Malvern
,
UK
). All measurements were performed with a wavelength of 670 nm, a detector angle of 90˚ and a temperature of 25˚C. The morphology of the micelles was observed by TEM using a JEM-100CX11 (JEOL,
Japan
).
2.9. Stability Studies of CMCS-g-β-CD・DMP Micelles
The stability study of CMCS-g-β-CD・DMP micelles was performed by DLS. The micelle dry powders (
0.01 g
) were dispersed in 10 mL PBS (pH 7.4) and placed for 30 days at room temperature. Their mean diameters and distributions before and after storage were measured.
2.10. pH-Sensitivity and Reduction-Response of CMCS-g-β-CD・DMP Micelles
The pH-sensitivity of the micelles was studied in media at various pH values. The micelle dry powders (
0.01 g
) were respectively dispersed in 10 mL PBS with various pH values. Then, these dispersions were incubated in a shake bed at a shaking speed of 60 rpm at 37˚C for 1 day. The mean diameters and distributions of the micelles were then measured by DLS.
The reduction-response of the micelles was investigated in media containing different GSH concentrations. The micelle dry powders (
0.01 g
) were respectively dissolved in 10 mL pH 7.4 PBS containing different GSH concentrations. Afterwards, the solutions were incubated in a shake bed at 37˚C and 60 rpm for 1 day. The mean diameters and distributions of the micelles were then determined by DLS.
2.11. Stimuli-Responsive Release of 6-MP from the Micelles
The stimuli-responsive release of 6-MP from the micelles was investigated in three different release media: 1) pH 7.4 PBS with 10 μM GSH; 2) pH 7.4 PBS with
5 mM
GSH; 3) pH 5.0 acetate buffer with
20 mM
GSH. The micelle dry powders (
0.01 g
) were dispersed in 10 mL release medium and straightway moved to a dialysis bag (MWCO 14,000). The dialysis bag was then immediately immersed in 40 mL release medium and oscillated in a shake bed at 60 rpm and 37˚C. At different time intervals, 2 μL of release medium was taken out and 2 μL of fresh medium was added. The medium taken out before was then analyzed by HPLC. The cumulative release rate of 6-MP was calculated using the following equation:
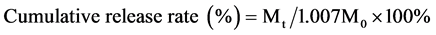 (1)
(1)
where Mt refers to the amount of 6-MP released at time t and M0 refers to the total amount of DMP in micelles.
2.12. Anti-Tumor Activity of CMCS-g-β-CD・DMP Micelles
MTT assay was employed to study the anti-tumor activity of CMCS-g-β-CD・DMP micelles. HeLa cells were seeded onto a 96-well plate in DMEM containing 10% FBS with a density of 1 × 104 cells/well. The cells were incubated at 37˚C for 1 day and exposed to 20 μL dispersions of micelles, and 20 μL dispersions of 6-MP and CMCS-g-β-CD with equivalent concentration. A 20 μL buffer was also added and used as a control. The cells were incubated for another 48 h. Then, 10 μL MTT solutions (5 mg/mL) were added into each well. After the cells were incubated for 4 h, 10 μL DMSO was added to each well to dissolve the formazan crystals. Finally, the optical densities (OD) were observed by a microplate reader at 570 nm. The inhibition ratios were calculated using following equation:
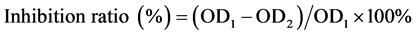 (2)
(2)
where OD1 refers to optical density of the control and OD2 refers to the optical densities of the cells exposed to the micelles, 6-MP and CMCS-g-β-CD.
3. Results and Discussion
3.1. Preparation and Structural Analysis of CMCS-g-β-CD・DMP
The synthetic route of CMCS-g-β-CD・DMP was shown in Figure 1. Firstly, CS and β-CD were modified with
![]()
Figure 1. Synthetic route and assembly process of CMCS-g-β-CD・DMP.
chloroacetic acid to obtain CMCS and CM β-CD. Then, CMCS-g-β-CD was obtained via the formation of amide bonds between the carboxyl groups of CM β-CD and the amino groups of CMCS. At last, CMCS-g- β-CD DMP was synthesized with DMP being partially embedding into the cavity of β-CD moiety.
The FT-IR spectra of CS, CMCS, β-CD, CM β-CD, DMP, CMCS-g-β-CD, the physical mixture of DMP and CMCS-g-β-CD (DMP content 9.37 wt%), 6-MP and CMCS-g-β-CD・DMP2 were shown in Figure 2. Compared with the spectrum of CS, the new characteristic bands at 1596 cm−1 and
1406 cm
−
1 appeared in the spectrum of CMCS were assigned to the symmetry and asymmetry stretching vibrations of COO-, indicating that carboxymethyl groups had been grafted onto chitosan. In the spectrum of CM β-CD, the characteristic band at
1720 cm
−
1 was ascribed to stretching vibration of carbonyl group (C=O). This band was not observed in the spectrum of β-CD, confirming that carboxymethyl group was grafted onto β-CD. The spectrum of CMCS-g-β-CD exhibited a characteristic band at
3426 cm
−
1 which was much higher than that of CMCS. This result was assigned to the existence of more hydroxyl groups in its structure resulting from grafting of CM β-CD. In addition, the characteristic bands of α-pyranyl vibration of CM β-CD at
946.2 cm
−
1 and β-pyranyl vibration of CMCS at
849.9 cm
-1 both appeared in curve e, which also confirmed that CM β-CD was grafted onto CMCS. Compared with the spectrum of 6-MP, the characteristic band of thiol groups at
2564 cm
−
1 disappeared in the spectrum of DMP, indicating that 6-MP had been oxidized to DMP. The two characteristic bands at
604 cm
−
1 and
638 cm
−
1 in the spectrum of DMP were assigned to the existence of 6-MP moiety. However, in the spectrum of CMCS-g-β-CD- DMP2, these two bands shifted to
646 cm
−
1 and
668 cm
−
1, while these two bands in the spectrum of the physical mixture remained unchanged. This result indicated the formation of the inclusion complex between CMCS-g- β-CD and DMP.
The DSC data shown in Figure 3 further supported the result. In the DSC profile of CMCS-g-β-CD, a broad endothermic peak could be observed, which was related to water evaporation. However, in the profile of the inclusion complex, the peak that represented water loses was vastly reduced in intensity and shifted to a lower temperature. This result suggested that a structure of lower water capacity was formed, because of the extrusion of water molecules from β-CD cavities, resulting from DMP being embedded into β-CD cavities [17] .
The 1H-NMR spectra of CMCS, CMCS-g-β-CD and CMCS-g-β-CD・DMP in D2O were shown in Figure 4. Compared with curve a, the new signals in the region of 5.0 - 5.2 ppm in curve b could be ascribed to the existence of D-glucopyranosyl residues of β-CD, suggesting that β-CD was successfully grafted onto CMCS. The signals at 3.86 - 3.90 ppm and 3.61 - 3.64 ppm in curve b were respectively assigned to two protons positioned
![]()
![]()
Figure 2. (A) FT-IR spectra of (a) CS, (b) CMCS, (c) β-CD and (d) CM β-CD; (B) FT-IR spectra of (e) CMCS-g-β-CD, (f) 6-MP, (g) DMP, (h) physical mixture of CMCS-g-β-CD and DMP (DMP content 9.37 wt%) and (i) CMCS-g-β-CD・DMP2.
![]()
Figure 3. DSC curves of (A) CMCS-g-β-CD・DMP2 and (B) CMCS-g-β-CD.
![]()
Figure 4. (A) 1H-NMR spectra in D2O of (a) CMCS, (b) CMCS-g-β-CD and (c) CMCS-g-β-CD・DMP; (B) Enlarge figures of the regions of (d) and (e).
inside the β-CD cavity, namely H
-3’
and H
-5’
. In curve c, the signals of H
-3’
and H
-5’
respectively shifted to 3.74 - 3.80 ppm and 3.51 - 3.54 ppm. This result was related to the ring-current of the aromatic ring that was embedded into β-CD cavity, suggesting the formation of an inclusion complex between CMCS-g-β-CD and DMP [18] . Furthermore, the strong signals at 8.28 ppm and 9.05 ppm in curve c could be ascribed to the DMP moiety which was located outside the β-CD cavity and the signals of the DMP moiety positioned inside the β- CD cavity shifted to 8.21 ppm and 8.99 ppm. These results confirmed the formation of the inclusion complex and the DMP was only partially embedded into the cavity of β-CD moiety.
3.2. Fabrication and Characterization of CMCS-g-β-CD・DMP Micelles
The fabrication procedures of the core-shell micelles were illustrated in Figure 1. Amphiphilic CMCS-g-β- CD・DMP was firstly synthesized and then underwent self-assembly to form a core-shell micelle. The TEM image of M
-2 in
pH 7.4 PBS was shown in Figure 5. It could be found that the micelles had a spherical core-shell configuration with a mean diameter of about 160 nm. Table 2 showed the DLS data of M
-2 in
pH 7.4 PBS. It could be easily found that diameter measured by DLS (194.7 nm) was larger than that (160 nm) by TEM. This might be attributed to the swelling of the micelles in water during DLS testing.
With DMP being partially embedded into the cavity of β-CD moiety, the non-covalently connected complex CMCS-g-β-CD・DMP obtained an amphiphilic macromolecule composed of a hydrophilic polymer backbone and hydrophobic DMP moieties that were located outside β-CD. After it dissolved in water, driven by the strong hydrophobic interactions, these hydrophobic drug moieties rapidly self-aggregated to form a hydrophobic core. And then, because of a high affinity with water, those hydrophilic polymer chains extended in the water and then frizzled to form a hydrophilic shell on the surface of the hydrophobic core. It should be noted that these inter- and/or intra-molecular hydrogen bonds in the hydrophilic shell also promoted the assembly process by increasing the stability of the shell [19] [20] .
![]()
Figure 5. TEM image of M
-2 in
pH 7.4 PBS.
![]()
Table 2. The mean diameters and distributions of the micelles in pH 7.4 PBS for their stability.
3.3. Stability of CMCS-g-β-CD・DMP Micelles
The stability study of the micelles was performed using DLS by placing the micelles in pH 7.4 PBS for 30 days. Before and after storage, their mean diameters and distributions were both determined and summarized in Table 2. It could be easily found that the micelles only had little diameter and polydispersity index (PDI) value change which indicated the micelles had good stability. This result might be ascribed to the formation of intra- and/or inter-molecular hydrogen bonds in the hydrophilic shell which made the micelles a non-covalently cross-linked hydrogel configuration.
3.4. pH-Sensitivity of CMCS-g-β-CD・DMP Micelles
The changes of the micelle diameters with pH alteration determined by DLS were shown in Figure 6. As the pH value increased from 3.0 to 6.0, the mean diameters of the three samples increased significantly and reached a maximum value at pH 6.0. This might be attributed to increasing of protonation of the carboxyl groups in the shells. After being protonated, the polysaccharide chains would become more hydrophilic and tend to repulse each other which finally led to a swell of the hydrophilic shell. As the pH value further increased to 8.0, however, a sudden decrease of particle diameters was observed, which could be attributed to the weaker electrostatic repulsion weakened by the charge screening effect of counter ions.
3.5. Reduction-Response of CMCS-g-β-CD・DMP Micelles
The reduction-response of the micelles was investigated by DLS in media containing various concentrations of GSH (0, 10 μM,
5 mM
and
20 mM
). As shown in Table 3, compared with the media without GSH, mean
![]()
Figure 6. pH-sensitivity of the micelles.
![]()
Table 3. The mean diameters and distributions of the micelles in pH 7.4 media with various concentrations of GSH for 24 h.
diameters and PDI values of the micelles in the buffer with 10 μM GSH changed little after incubated for 24 h. As the GSH concentration increased to
5 mM
, it could be easily found that an obvious increase appeared in both particle diameters and PDI values. Furthermore, a larger increase could be observed when the GSH concentration increased to
20 mM
. These data indicated that the particle diameters and PDI values of the micelles increased with the increase of GSH concentration in aqueous solution.
3.6. Stimuli-Responsive Release of 6-MP from the Micelles
The stimuli-responsive release of 6-MP from the micelles was investigated with M-2. As shown in Figure 7, 6-MP cumulative release from the micelles was about 27.3 wt% in pH 7.4 PBS with 10 μM GSH (mimicking the blood circulation) after 48 h. When the GSH concentration increased to
5 mM
(mimicking the intracellular environment of normal cell), it showed that 6-MP release reached over 59 wt% after 48 h. In pH 5.0 medium with
20 mM
GSH (mimicking the intracellular environment of tumor cell), it exhibited a much rapider release to over 22 wt% in initial 1 h, and over 46 wt% in 3 h, and finally reached over 88 wt% after 48 h. These data indicated that the CMCS-g-β-CD・DMP micelles were likely to remain stable in blood circulation, and could achieve selective and fast drug release responding to the higher GSH concentration and lower pH value in tumor cells.
The release mechanism of hydrophobic 6-MP was highly dependent on diffusion process and degradation of the micelles. 6-MP could be released from DMP through two procedures. The first was that via an exchange reaction between DMP and GSH, one 6-MP was released in free form and the other was released in form of 6-MP-GSH. After that, 6-MP-GSH could again release free 6-MP by an exchange reaction with another GSH [7] . In pH7.4 PBS with 10 μM GSH, drug release was likely to obey the diffusion mechanism and was controlled by the swelling degree of the micelles. In pH 7.4 PBS with
5 mM
GSH, the release of 6-MP was controlled by the cleavage of disulfide bonds and followed the degradation mechanism. In pH 5.0 acetate buffer with
20 mM
GSH, the fast release of 6-MP was mainly because the disulfide bonds in the core were splitted by the GSH of high concentration in the medium. In meantime, we discovered that the particle size of M
-2 in
pH 5.0 medium was a bit larger than that in pH 7.4 medium. This might be conductive to exposing the disulfide bonds in the hydrophobic core to extern GSH. These two reasons finally led a rapid and nearly complete release of 6-MP.
The effect of drug content on the release behavior of the micelles was investigated in pH 7.4 PBS with
5 mM
GSH. As shown in Figure 8, 6-MP cumulative release rate form M-1 was about 22 wt% in initial 3 h and about
![]()
Figure 7. Drug release profiles from M
-2 in
pH 7.4 PBS with 10 μM and
5 mM
GSH, and pH 5.0 acetate buffer containing
20 mM
GSH in 48 h.
50.1 wt% after 48 h. The release rate from M-2 reached about 28 wt% in initial 3 h and over 59 wt% after 48 h, whereas M-3 reached about 29 wt% in initial 3 h and over 69 wt% after 48 h. The data showed the release rate increased with the increase of drug content in CMCS-g-β-CD・DMP micelles.
3.7. Anti-Tumor Activity of CMCS-g-β-CD・DMP Micelles
The cytotoxicity of the CMCS-g-β-CD・DMP micelles was evaluated in HeLa cells using MTT assay. As shown in Figure 9, free 6-MP was employed as a positive control and blank CMCS-g-β-CD was employed as a negative control. The negative control experiment revealed that CMCS-g-β-CD was nearly non-toxicity to HeLa cells, indicating the polymer had good biocompatibility. The M-2, however, exhibited a dose-dependent cytotoxicity
![]()
Figure 8. Drug release profiles from the micelles of various DMP contents in pH 7.4 PBS with
5 mM
GSH in 48 h.
![]()
Figure 9. Cell inhibition ratio of in vitro cytotoxicity of CMCS-g-β-CD, CMCS-g-β-CD・DMP micelles and free DMP at various concentrations against HeLa cells.
for HeLa cells which was comparable to that of free 6-MP after incubation for 48 h. This result should be attributed to the fast release of 6-MP from M
-2 in
intracellular environment of HeLa cells, which confirmed the pH/redox responsive micelles could be used for controlled release of 6-MP in tumor cells.
4. Conclusion
In summary, we had shown that: 1) an inclusion-interaction assembly strategy was used to construct novel pH/ redox responsive micelles, with the drug as the core and the polymer as the shell; this strategy could achieve integration of drug-loading and self-assembly, avoiding the multiple steps used in covalent-bond method; 2) TEM photograph confirmed that the micelles had a spherical core-shell structure; 3) investigations by DLS showed that the micelles were stable in water and had a narrow size distribution as well as a good pH/redox sensitivity; 4) in vitro drug release showed a selective release in pH 5.0 medium containing
20 mM
GSH; 5) in vitro cytotoxicity test showed that the micelles had a dose-dependent toxicity for HeLa cells, indicating that the micelles held great potential for controlled release of 6-MP in tumor cells.
Acknowledgements
This work was financially supported by Natural National Science Foundation of China (Nos. 51373130, 51273156 and 31300791).
NOTES
*Corresponding authors.