1. Introduction
It has been well recognized that TiO2 has interesting properties to fabricate photo-induced applications because of the highly efficient surface photo-catalytic reactions and the hydrophilicity [1] [2] . In addition, the materials can be also used as optoelectronic devices using anti-reflection coating such as solar-cell, optical filter and so on, because of the high refractive index [3] . In electronics, TiO2 with high dielectric constant and relatively high energy band gap was tried to use for gate-dielectrics in MOS-transistor [4] , and the semiconductor has been recently expected to be applied for transparent conduction oxide (TCO). For the application as TCO, the anatase- phase is probably more useful than the rutile-phase because energy gap of the anatase (3.2 eV) is wider than that of rutile (3.0 eV) and TiO2 can be crystallized in anatase-phase at temperature far lower than that required to form rutile-TiO2. Further, the conductive TiO2 with notably high resistance against acid and alkaline solutions has interesting potentials to fabricate wet-type solar-cells such as dye sensitized solar cell [5] , chemical sensors etc. However, it is considered that the conductivity control is more difficult than the other TCO such as ITO, Sn2O and ZnO since d-orbital contributes to forming TiO2. Recently, physical vapor deposition such as laser-ablation and reactive sputtering with the post-annealing in reduction ambient have been applied to control the conductivity by using Nb as a donor-dopant and achieved fabrication of highly conductive TiO2 layer [6] [7] . In the results, it is seemed to be required to enhance the electronic activation of the donor [8] , in which Ti3+ reduced from Ti4+ probably plays an important role of the conduction in TiO2. Although precise control is required to deposit semiconductor layers, chemical vapor deposition (CVD) has great advantages for step coverage on three-dimensional surface. In the CVD process, suitable gas-source as preliminary precursor is fundamentally required with control of the deposition condition. For the deposition of TiO2 layer, titanium-tetra-iso-propoxide (TTIP:Ti(O-i-C3H7)4) is one of suitable metalorganic precursors. The deposition feature including TTIP-dissoci- ation scheme has been studied on the crystallographic properties, in which it has been recognized that the thermally dissociated species can be crystallized into the anatase-phase around 400˚C [9] . However, the conductivity control has not been achieved for TiO2 layer by CVD. Previously, we demonstrated low pressure chemical vapor deposition (LPCVD) of Nb and F co-doped TiO2 layer using TTIP and NbF5 in O2-ambient [10] , which was resulted in drastic decrease of resistivity to 0.2 Ω∙cm in comparison to the resistivity of undoped layer beyond 100 Ω∙cm. Here, it was shown by the XPS results that F substituted to the O-site contributes to the reduction of resistivity but CHx-F by reaction between alcohol ligands in TTIP and F, in which the fluorination occurred by oxidation of NbF5. O2 gas has been commonly used to deposit anatase-TiO2 layer by CVD process aiming at reduction of oxygen-deficiency and carbon-impurities; however, the effect for removal carbon impurity is not expected to the deposition using TTIP at such low temperature around 400˚C because the precursor is mainly dissociated in the O-R bond of TTIP without oxidation of the alcohol ligands [11] . In contrast, anatase-TiO2 can be fabricated in H2 by LPCVD using TTIP as previously reported [12] , in which the resistivity was comparably low to Nb and F co-doped layer deposited in O2 but including structural disordering and significant optical absorptions including Urbach tail in UV-Vis region.
In this paper, LPCVD of TiO2 layer using TTIP with NbF5 is demonstrated in H2-ambient, and the deposition feature and the resistivity are shown with the optical property in UV-Vis region.
2. Experimental
A bell-jar type reactor with the base pressure under 1 × 10−3 Pa by a combination exhaust system consists of a diffusion pump and a rotary pump was used for LPCVD of titanium-oxide. Titanium tetra-iso-propoxide (TTIP, Ti(O-i-C3H7)4: 99.7%-purity) was used as preliminary precursor for the deposition after purification in vacuum. Details of the apparatus configuration and the purification sequence of TTIP were already shown elsewhere [10] . TTIP vaporized from the liquid charge cell at 65˚C was introduced into the reactor controlling the supply rate by a variable valve with monitoring the reactor pressure by Schulz gage. In this work, the pressure of TTIP was fixed at 1.5 × 10−1 Pa during TiO2 deposition. High purity (99.99999%-purity) H2 gas was also introduced into the reactor through a mass-flow controller calibrated for H2 gas in ranging from 0.75 to 8.3 sccm, in which the reactor pressure monitored by Schulz gage was varied from 1.8 × 10−2 to 1.5 × 10−1 Pa. TTIP and H2 were simultaneously introduced into the reactor through individual gas inlets and TiO2 layer was deposited at pressure ranging from 0.15 to 0.30 Pa. Niobium pentafluoride powder (NbF5: 98%-purity) as donor-dopant was charged in a crucible consists of boron-nitride (BN) and then thermally evaporated after purification for 5 hrs in vacuum. Evaporation rate of the dopant was estimated from the total evaporation mass evaluated by an electronic weight scale during the deposition. 1 mm-thick optical-flat quartz plate used as substrate was mounted on a substrate holder after chemical cleaning by organic solvents. Temperature of the substrate holder and the BN crucible were monitored by K-type thermo-couples and controlled by resistive heating with PID-systems using.
Thickness of the layer was checked by a surface profiler (Veeco, DEKTAK150). Resistivity was evaluated by Van Der Pauw (VDP) method using symmetric four ohmic contacts of Indium-dots. UV-Vis optical transmission spectra were obtained by UV-Vis spectrometer (OCEAN OPTICS: USB-2000) using halogen lamp as a light source.
3. Results and Discussion
3.1. Deposition Feature
3.1.1. Dependence of Deposition Rate on the Temperature
Figure 1 shows deposition rate of undoped layer in H2-ambient (black-circle) or O2-ambient (open-circle) and Nb-F doped layer (red-circle) in H2-ambient at various deposition temperatures in Arrhenius plot, in which the gas flow rate of H2 or O2 into the reactor was 4.2 sccm and NbF5 was evaporated from crucible with the rate about 0.05 mg/min for the deposition of doped layer. Deposition rates of the undoped layers were increased with the temperature and then saturated to the rate about 8 nm/min at the temperature above 360˚C, in which the activation energy of 228 kJ/mol in H2 was clearly larger than that of 138 kJ/mol in O2. Here, it is considered that TTIP-dissociation in H2-ambient is not owing to chemical reaction on the deposition surface because the higher activation energy is similar to activation barrier of 238 kJ/mol to form Ti(OC3H7)3(OH) through monomolecular dissociation of TTIP calculated by quantum chemical calculations using B3LYP. The surface chemical states have not been identified but it is expected that the surface is terminated by hydroxyls which can be formed during TTIP-dissociation and/or by hydrogenation of oxygen at the surface [12] . On the other, when NbF5 was simultaneous supplied during the deposition in H2, the deposition rate was increased with the activation energy of 120 kJ/mol and then saturated to 18 nm/min above 380˚C. The activation energy and the saturation temperature were smaller and higher than that for the undoped layer in H2-ambient respectively, which was resulted in higher saturated deposition rate. The results clearly indicate TTIP-dissociation in H2 was promoted by NbF5. Here, it is difficult to recognize reduction of the activation energy by NbF5 supply in H2 is due to fluorination of TTIP because the fluorination reduces the deposition rate as shown previously for the deposition in O2-ambient [10] . Further, it is mentioned sticking probability of TTIP on the deposition surface was increased by NbF5 contribution because the saturated deposition rate at high temperature was increased by NbF5 supply. As the results, it should be concluded that the TTIP-dissociation was promoted by reactive site on the surface contributed by NbF5.
3.1.2. Dependence of Deposition Rate on NbF5 & H2 Supply Rate
Deposition feature of the doped layer was also influenced by NbF5 and H2 gas supply rate. Figure 2(a) shows dependence of TiO2 deposition rate at 380˚C in H2 with the flow rate of 0.75 (open-circle), 4.2 (solid-circle) and 8.3 sccm (solid-triangle) on NbF5 evaporation rate from crucible, where TTIP supply rate was same for the depositions. The deposition rate was linearly increased and then gently decreased with NbF5 evaporation rate de-
![]()
Figure 1. Deposition rate of undoped (black-circle) and Nb-F doped (red- circle) layers in H2-ambient and undoped layers in O2-ambient (open-circle) at various deposition temperatures, where the layers were deposited at 0.25 Pa with H2 or O2 flow rate of 4.2 sccm. NbF5 was evaporated with the rate about 0.05 mg/min for the deposition of doped layer.
pending on H2 flow rate. It is easily expected that F play an important role for TTIP-dissociation but not the fluorination. Further, it is difficult to recognize that the significant increase of deposition rate was due to the reaction between TTIP and F in vapor phase because the density of NbF5 was far lower than the TTIP, in which the NbF5/TTIP supply ratio can be roughly estimated as a few % from the TiO2 deposition rate and the NbF5 evaporation rate with taking the reactor structure into account. Therefore, it should be discussed the TTIP-dissociation contributed by F on the deposition surface as shown in the next section. It is however considered the excessive NbF5 fluorinates TTIP, which is resulted in decrease of the deposition rate. Since structural disordering in the layer is enhanced by the fluorination of alcohol ligands of TTIP as previously suggested [10] , the excess NbF5- supply on the deposition surface is insufficient for the deposition. The deposition rate linearly increased with NbF5 evaporation rate was decreased with H2 flow rate as shown in Figure 2(b), in which incremental rate of deposition rate due to NbF5 evaporation rate is shown for H2 flow rate. The result suggested density of the reactive species for TTIP-dissociation on the deposition surface is decreased with H2 density on the surface, which can be recognized that surface F is non-activated as HF by H.
3.1.3. Expected Deposition Scheme on Deposition Surface
Previously, it was reported that HF interacts to hydroxyl groups and oxygen on TiO2 surface by hydrogen- bonding in weak acidic HF solution and hydroxyl groups on TiO2 surface is easily exchanged to F in strong acidic HF solution [13] . Although HF is not preliminary supplied on the deposition surface in the LPCVD pro- cess, it is expected that Ti-F is formed with NbF4OH by the reaction between NbF5 and hydroxyls on the surface, then HF is produced after TTIP is adsorbed at the reactive site of surface F in Ti-F. According to the reaction, a possible scheme for the deposition with NbF5-supply can be depicted in Figure 3. When NbF5 is supplied on surface hydroxyl groups in Scheme 1, the OH is exchanged to F with formation of NbF4OH (Scheme 2). The next coming TTIP adsorbed to the surface F is dissociated with forming HF and propene, and then the partially dissociated TTIP is coordinated to surface Ti (Scheme 2 & Scheme 4) as O-Ti-(OR)3 which then form oxo-bridging between the neighbors via the thermal dissociation. Here, the produced HF interacts to the neighbor hydroxyl groups (≡Ti-OH) and oxygen (Ti-O) (Scheme 4), then ≡Ti-F is formed with product of H2O (Scheme 5), in which the reactions from Scheme 2 to Scheme 5 are sequentially processed on the deposition surface. As previously discussed, TTIP-dissociation is owing to the monomolecular thermal process due to the low sticking probability on the hydrogen-terminated surface (Scheme 1), which is resulted in the significant increased activation energy for the deposition rate in H2-ambient [12] . In contrast, it is expected the surface F with high electron negativity acts as catalyst for partial dissociation of TTIP (from Scheme 3 to Scheme 4). As the result, activation energy for the deposition rate is significantly decreased by NbF5-supply as shown in Figure 1 and the deposition rate is increased with the density of F (NbF5 evaporation rate) as shown in Figure 2(a), whereas the of F-density is far lower than that of TTIP. It is noted that hydrogen supplied as H2 gas acts to form HF on the surface before TTIP is adsorbed to the surface F in Scheme 2 or Scheme 5, which is resulted in decrease
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) Dependence of deposition rate on NbF5 evaporation rate in H2 flow rate of 0.75 (solid-circle), 4.2 (open-circle) and 8.3 sccm (solid-triangle); (b) Dependence of incremental rate for deposition rate by NbF5 in the evaporation rate below 0.05 mg/min on H2 flow rate.
![]() (Scheme 1) (Scheme 2) (Scheme 3) (Scheme 4) (Scheme 5)
(Scheme 1) (Scheme 2) (Scheme 3) (Scheme 4) (Scheme 5)
Figure 3. An expected dissociation scheme for TTIP and NbF5.
of the deposition rate with increasing the H2 flow rate as shown in Figure 2(b). On the other, excessively supplied NbF5 probably causes fluorination of alcohol ligands in Scheme 4 or Scheme 5, which is insufficient to control property of the layer because significant structural disordering is introduced into the layer.
3.2. Electric Property
3.2.1. Dependence of Resistivity on Deposition Temperature
Figure 4 shows resistivity of undoped (black-circle) and doped (red-circle) layers with the thickness about 200 nm deposited at various temperatures in H2 with the flow rate of 4.2 sccm, where NbF5 evaporation rate was fixed at 0.05 mg/min for the doping. Resistivity of the undoped layer was decreased with increasing the deposition temperature according to the activation energy of 244 kJ/mol but increased beyond 360˚C. In case of the doped layer, the resistivity higher than the undoped layer at low temperature was also decreased with the deposition temperature below 380˚C with larger activation energy (319 kJ/mol) than the undoped layer. As the result, resistivity of the doped layer deposited at temperature beyond 370˚C was more reduced than the undoped layer. We previously speculated that Ti3+ is formed in the layer with oxygen-vacancy by desorption of hydrogen from H-Ti≡ formed in H2-ambient, in which the resistivity is decreased with the temperature according to the dissociation energy of H-Ti≡ [12] . It is however difficult the same scheme for the layer deposited by NbF5 supply in H2 because the activation energy was larger than the dissociation energy. When NbF5 is simultaneously supplied to the deposition surface, F and Nb substituted to O-site and Ti-site are expected to act as relatively shallow donors in TiO2 respectively, where the F generates Ti3+ without O-vacancies and the Ti3+ forms Ti-3d1 band [14] . In contrast, interstitial F and anti-site Nb reduce the carrier density because such defects act as acceptors. In case of the doping in O2-ambient, F is substituted to the O-site but the crystallinity was significant degraded by fluorination of TTIP due to oxidation of NbF5 [10] . In contrast, it is considered the fluorination can be prevented in H2-ambient because of removal the oxidant for NbF5 but Nb reduced from NbF5 by hydrogen maybe substitute the O-site. Indeed, the activation energy of 319 kJ/mol is close to the bond dissociation energy of Nb-Ti (302 kJ/mol) [15] . On the other, the resistivity was increased at high temperature beyond 360˚C for the undoped layer and 380˚C for the doped layer. It is interesting the deposition rate was limited by TTIP-supply rate at such high temperatures, which indicates the most efficient doping can be achieved at the highest temperature in surface reaction limited region for the deposition rate.
3.2.2. Dependence on NbF5 & H2 Supply Rate
Figure 5(a) shows resistivity of the layer as a function of NbF5 evaporation rate in H2 with the flow rate of 0.75 (open-circle), 4.2 (solid-circle) and 8.3 sccm (solid-triangle), where the layers were deposited with the thickness about 200 nm at 380˚C. The results can be recognized not only NbF5 evaporation rate but also H2 flow rate affects to the doping feature. For the NbF5 evaporation rate, the resistivity was decreased with increasing the evaporation rate but the feature was different in the high evaporation rate. Since the deposition feature was disturbed in the high NbF5 evaporation rate as shown in Figure 2(a), it is not easy to discuss the dependence of resistivity on the doping in the high NbF5 evaporation rate. In contrast, the resistivity in the low NbF5 evaporation rate is uniquely decreased with the evaporation rate, where the dependence should be discussed for NbF5 density on the deposition surface. The NbF5 supply rate on the deposition surface (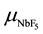 ) is proportional to [NbF5]/DR, because the density is increased with NbF5 evaporation rate ([NbF5]) and decreased with deposition rate of the layer (DR). Figure 5(b) shows resistivity of the doped layer for [NbF5]/DR in log-log scale. Resistivity of the doped layer (ρdoped) was decreased with [NbF5]/DR, which was resulted in the relationship of ρdoped ~ (
) is proportional to [NbF5]/DR, because the density is increased with NbF5 evaporation rate ([NbF5]) and decreased with deposition rate of the layer (DR). Figure 5(b) shows resistivity of the doped layer for [NbF5]/DR in log-log scale. Resistivity of the doped layer (ρdoped) was decreased with [NbF5]/DR, which was resulted in the relationship of ρdoped ~ (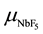 )n. If
)n. If
![]()
Figure 4. Resistivity of undoped (black-circle) and Nb-F doped (red-circle) layers in H2-ambient at various deposition temperatures, where the resistivity was evaluated for the layers with the thickness about 200 nm.
the donor was simply increased in the density with , the resistivity should be decreased in the factor n of −1. In contrast, the factor n less than −1 and beyond −1 suggests the crystallinity including the grain boundary was improved and degraded with the donor density, respectively. In the result of Figure 5(b), the factor for the deposition in H2 with the flow rate of 0.75, 4.2 and 8.3 sccm was −0.94, −1.7 and −0.45 respectively. Figure 5(c) shows variation of the factor n for the H2 flow rate, in which the factor n was decreased with the H2 flow rate but notably increased by excess H2 flow rate. It should be mentioned here that the factor n was less than −1 for the deposition with H2 supply rate of 4.2 sccm. The interesting result suggests the doping with sufficient H2 supply not only increased donor density but also improved the crystallinity. In previous report, we speculated oxygen-vacancies increase resistivity of the doped layer [10] , in which the defects probably form complex centers with deep levels. In contrast, it is expected that F doping into O-site in TiO2 achieves to reduce the O-vacancies in the density and creates Ti3+ with shallow donor level. Of course, the doping feature is owing to the dissociation scheme of the dopant. The result of the factor n dependent on H2 flow rate indicates sufficient hydrogen supply is required for the efficient doping but excess hydrogen is resulted in invalidation of the doping. The insufficient doping in excess H2 is probably caused by anti-site Nb which is introduced by species excessively reduced from NbF5 as speculated in the Section 3.1.1.
, the resistivity should be decreased in the factor n of −1. In contrast, the factor n less than −1 and beyond −1 suggests the crystallinity including the grain boundary was improved and degraded with the donor density, respectively. In the result of Figure 5(b), the factor for the deposition in H2 with the flow rate of 0.75, 4.2 and 8.3 sccm was −0.94, −1.7 and −0.45 respectively. Figure 5(c) shows variation of the factor n for the H2 flow rate, in which the factor n was decreased with the H2 flow rate but notably increased by excess H2 flow rate. It should be mentioned here that the factor n was less than −1 for the deposition with H2 supply rate of 4.2 sccm. The interesting result suggests the doping with sufficient H2 supply not only increased donor density but also improved the crystallinity. In previous report, we speculated oxygen-vacancies increase resistivity of the doped layer [10] , in which the defects probably form complex centers with deep levels. In contrast, it is expected that F doping into O-site in TiO2 achieves to reduce the O-vacancies in the density and creates Ti3+ with shallow donor level. Of course, the doping feature is owing to the dissociation scheme of the dopant. The result of the factor n dependent on H2 flow rate indicates sufficient hydrogen supply is required for the efficient doping but excess hydrogen is resulted in invalidation of the doping. The insufficient doping in excess H2 is probably caused by anti-site Nb which is introduced by species excessively reduced from NbF5 as speculated in the Section 3.1.1.
3.2.3. Optical Property
Figure 6(a) shows transmission spectra in UV-Vis region of undoped layer (black-line) and Nb-F doped layer (red-line) deposited in H2, in addition to the spectrum of Nb-F doped layer deposited in O2 (blue-line) [9] . The layers were deposited with the thickness about 200 nm by the condition optimized to reduce the resistivity and
![]()
![]() (a) (b)
(a) (b)
Figure 6. (a) Transmission spectra and (b) the Tauc-plot in (αhν)1/2 vs. photon energy of undoped layer (black-line) and Nb-F doped layer (red-line) deposited in H2, and Nb-F doped layer (blue-line) deposited in O2, where the layers were deposited with the thickness about 200 nm by the optimized conditions.
resulted in the resistivity of 0.2, 0.05 and 0.2 Ω∙cm for the undoped and the Nb-F doped layers in H2 and the Nb-F doped layer in O2 respectively. Here, the spectrum of undoped layer in O2 was similar to the blue line. As previously reported, the undoped layer in H2 showed broad optical-absorptions around 3.0 eV and 2.2 eV due to deep levels concerned with oxygen-vacancies and Ti3+ [10] respectively. In contrast, although significant multi-reflection was included, such absorptions were remarkably prevented in the spectrum of the doped layer deposited in H2. Especially, the absorption around 3.0 eV was obviously decreased in the doped-layer, which indicated density of oxygen-vacancy in the doped layer was notably reduced in comparison to the undoped layer. It is not difficult to anticipate O-vacancy is easily induced into the oxide in H2-ambient but is not originated from the reduction of TiO2 in the CVD-process. The defect is probably formed by TTIP-dissociation via monomolecular thermal dissociation as suggested previously [12] . In the case of simultaneous supply of NbF5, the dissociation scheme is quite differed as shown in Figure 3, in which TTIP can be chemically dissociated at the reactive site of F on the deposition surface. As the result, the deposition can be performed with preventing O-vacancy by the dissociation supported by the surface F. In addition, F-doping into the O-site may reduce the density of O-vacancy. It is noted that the optical transparence of the Nb-F doped layer deposited in H2 was better than the doped layer in O2. Commonly, O2-gas has been used for CVD of TiO2 to improve the property aiming at removal oxygen-deficiency and residual carbon-related impurities. However, the results for optical absorption revealed H2 simultaneously supplied with NbF5 is more effective than O2 to reduce optical absorption due to deep-levels especially originated from oxygen-vacancies. It is believed the reduction of the deep-level density was come from the sufficient TTIP-dissociation on the deposition surface and F substituted to the oxygen-site. On the other, structural disordering causes the optical band gap narrowing due to absorption tail below the fundamental absorption edge which is referred as Urbach tail [16] . Figure 6(b) is Tauc-plot in (αhν)1/2 vs. photon energy, where α, h and ν is absorption coefficient, Plank constant and wavenumber respectively. Optical band gap of 3.0 eV for the undoped layer in H2 was narrower than 3.2 eV for anatase-TiO2. In contrast, the band gap energy of 3.27 eV for the doped layer was in good agreement with 3.26 eV for anatase-TiO2 [17] , which indicated structural disordering in the doped layer was significantly improved as compared with in the undoped layer. Here, the band gap energy of 3.20 eV for the doped layer in O2 was slightly large as compared with the energy of 3.16 eV for the undoped layer in O2 [12] , which suggested the structural disordering can be improved also in O2 by NbF5-supply. However, the larger energy of 3.26 eV for the doped layer in H2 indicated NbF5-supply in H2 is more effective than that in O2.
4. Conclusion
Low-pressure chemical vapor deposition of TiO2 layer was demonstrated in H2-ambient by using TTIP with NbF5. Activation energy for the deposition rate was significantly reduced to 120 kJ/mol by NbF5-supply in comparison to the energy as high as 228 kJ/mol for the deposition without NbF5. In addition, the deposition limited by the surface reaction of TTIP was performed at temperature below 380˚C, whereas the deposition without NbF5-supply above 360˚C was limited by the TTIP-supply rate. The deposition rate at 380˚C was linearly increased with NbF5-supply rate but slightly decreased by the excess supply. An expected scheme for TTIP and NbF5 dissociation suggested that F dissociated from NbF5 by reaction between NbF5 and OH on the deposition surface acts as catalyst for TTIP. Resistivity of the layer was decreased with increasing deposition temperature in the surface reaction limited region for the deposition rate, in which the activation energy of 319 kJ/mol is higher than 244 kJ/mol for the layer deposited without NbF5. The results for resistivity dependent on NbF5 and H2 supply rates indicated that H2 is required to achieve efficient doping but excess H2 increases the resistivity. As the result, TiO2 layer with the resistivity as low as 5 × 10−2 Ω∙cm could be fabricated by LPCVD using TTIP and NbF5 in H2-ambient. Further, optical transmission spectra showed that the density of optical absorption centers in UV-Vis region was notably decreased in the low resistive layer deposited in H2 with NbF5-supply as compared with the undoped layer and the Nb-F doped layer in O2, in addition to increase of the optical absorption edge energy. Such results clearly indicated that low-resistive TiO2 layer with high transparency can be deposited by the presented process using TTIP with NbF5 in H2-ambient.
NOTES
*S. Hatakeyama now belongs to Hitachi Industry & Control Solutions, Ltd.