Adsorption and Transport of Ciprofloxacin in Quartz Sand at Different pH and Ionic Strength ()
1. Introduction
In large-scale livestock farming, the increasing consumption of various antibiotics including quinolones, tetra- cyclines, sulfonamides, etc. has become an irreversible trend around the world [1] [2] . However, most antibio- tics cannot be completely assimilated into the animal body, and 40% to 90% of the antibiotic dose will be ex-
creted with the feces and urine in the form of precursor or metabolite [3] . As a result, the final antibiotics in animal faeces may reach tens of mg/kg or more [4] [5] , which finally go into the soil environment through the application of agricultural manure. Relevant studies indicate that the amount of antibiotics entering into the soil is comparable to the pesticide application rate [6] . Antibiotics not only affect soil fertility and the safety of agricultural products, but also pollute water by surface runoff and leaching, ultimately threatening animal and human health. Nowadays, the antibiotics directly entering into the soil environment through the application of agriculture manure have become a potential risk of environmental pollution [7] - [9] . Therefore, the antibiotic pollution has become an important environmental organic pollutant and meanwhile a hotspot in environmental science.
Ciprofloxacin (CIP) is a quinolone antibiotic widely used in livestock and poultry breeding industry because of its broad-spectrum antibacterial and efficiency. Along with its popularization, various environmental problems also appear, and have attracted more and more attentions from domestic and foreign scholars. However, current research on CIP mainly focuses on the clinical application of animal diseases, animal in vivo pharmacokinetics, drug residues in animal products, etc. [10] - [13] , but seldom concerns the transport behaviors of CIP after it enters into the environment from the perspective of ecological security. There are less research on CIP adsorption and transport in the soil, and the situation is even worse in China.
Prior to this study, the CIP adsorption characteristics in the soil at different pH and ionic strength have been studied using the measurement methods including HPLC, MS, GC, etc. [14] . However, due to the restriction of current laboratory conditions, the samples were analyzed by UV spectrophotometer, and the experimental data were not ideal. There were probably two reasons: Firstly, the soil contained a lot of organic matter that interfered with the analytical measurement, so that the peaks were not obvious at 273 nm wavelength under UV detection conditions; secondly, the mineral composition in the soil, introduced by inappropriate centrifugal filtration, led to the filtrate turbidity, which resulted in inaccurate measurements. At the beginning, it was suspected that the filtration operation was not good enough, but the experimental data were not improved even with qualitative and quantitative filter paper. Further, after EDTA-2Na salt was added in the filtrate as reagent, the results of UV spectral scanning were not improved yet. Considering UV could not accurately measure the CIP in the soil, ICP was tried to measure the CIP in the soil indirectly through measuring the zinc content by creating CIP― zinc complexes in the sample. However, the complex concentration was too low to produce obvious precipita- tion, and no meaningful experimental data were obtained.
Therefore, considering the complexity of the soil system, in order to analyze the adsorption characteristics of ciprofloxacin under a single factor, the quartz sand of low reactivity was taken as a medium to explore the CIP absorption in quartz sand at different pH and ionic strength, and the mixed displacement experiments were car- ried out indoors to demonstrate the vertical transport characteristics of CIP in the soil profile. This study can provide a scientific basis for further revealing adsorption mechanism in the soil and the ecological risk assess- ment and management, and meanwhile plays an important role in the rational use of ciprofloxacin in animal husbandry.
2. Materials and Methods
2.1. Instruments and Reagents
Instruments: Oscillator THZ-92A, Haibo Motion Industries Limited Medical Equipment Factory; Centrifuge TDL-4A, Heidfeld Pradesh Analytical Instruments Co., Ltd.; UV spectrophotometer, Beijing Lebo Tektronix Instrument Co., Ltd.; pH meter PHS-3C, Shanghai Jing Branch Instrument Co., Ltd.; Soil column with a height of 10 cm and an inner diameter of 5 cm, own product made of plexiglass; Peristaltic pump, BT100-1F, Baoding Lange cross-flow pump, Ltd.; Automatic part collection, BSZ-100, Shanghai Huxi Analytical Instru- ment Factory.
Reagents: CIP reference (purity 98.0%, Tokyo Kasei Kogyo Co., Ltd.); other chemical reagents (analytical grade); test water, crystal-D ultrapure water.
2.2. Test Materials
Quartz sand is a kind of hard and wear-resistant quartzte mineral with stable chemical properties. Its main mineral component is SiO2 (99.5 wt%), along with tiny iron oxide (<0.02 wt%), clay, mica and organic impurities. Quartz sand is milky white or colorless translucent solid with the relative density of 2.65. It is insoluble in acid but slightly soluble in KOH solution. The quartz crystals own porous structure, which can absorb various par- ticles or molecules. In general, the adsorption and chemical properties of SiO2 surface are mainly determined by the surface hydroxyl groups, and CIP is just adsorbed on the surface of the medium by forming hydrogen bonds with hydroxyl groups [15] . Before each experiment, the quartz sand was sieved over 20 - 30 mesh sieve, and then soaked in 0.01 mol/L NaOH for 24 h and 0.01 mol/L HCl for 24 h successively to remove the metal oxide on the surface. After soaked in NaOH or HCl solution, the quartz sand was washed with distilled water until the pH was close to neutral. After dried at 105˚C, the spare test materials were obtained [16] .
2.3. Experimental Methods
2.3.1. Adsorption Isothermal Experiment
In this experiment, eighteen 50 mL centrifuge tubes were divided into three groups, and 1 g sample of quartz sand was added into each tube respectively. Then, CIP-KCl solutions of different concentrations were prepared using 0.01 mol/L KCl solution as supporting electrolyte, and the CIP concentrations in the same group were 0, 10, 20, 30, 40, 50 mg/L respectively. pH value of the three groups was adjusted to 5, 6 and 7 respectively, and 20 mL CIP solution with different pH was added into each centrifuge tube respectively according to grouping requirements. The solution was sealed and shaken at 25˚C for 24 h, and then centrifuged. The supernatant was removed by filtration and the filtrate was diluted by 5 times. The CIP concentration of the supernatant was measured by UV spectrophotometer, and then the adsorption amount was calculated. Three replicates were done for the above operations, and the blank (CIP solution without soil) was set. In order to avoid oscillations during the photo degradation of antibiotics, the whole process was carried out in the dark.
2.3.2. Effects of Ionic Strength on CIP Adsorption
Monovalent metal ions such as Na+ and K+ have an impact on the adsorption by competing adsorption sites with antibiotics of cationic state or zero valence state [17] . With the same experimental method described above, the pH of CIP was keep as 6 and the ionic strength were 0.01, 0.05 and 0.1 mol/L, respectively. Then, the CIP concentration in solution was measured and the adsorption capacity was calculated. The measurements above were repeated three times.
2.3.3. Soil Column Outflow Experiments
To analyze the effects of pH and ionic strength on CIP transport in quartz sand, the soil column outflow experi- ments were carried out. The experiments were conducted indoors in one-dimensional saturated soil column with a height of 10 cm and a diameter of 5 cm. The washed and dried quartz sand was filled into the soil column in 5 times, and the soil column was compacted by plastic compactor each time to ensure uniform distribution of quartz sand particles. Meanwhile, some cotton wool was added to the higher and lower ends of each interface in the soil column in order to prevent quartz sand particles from clogging the water hole. At the each end there was a layer of filter paper which ensured that the solution could be uniformly penetrated into the quartz sand [18] . Taking a beaker and a peristaltic pump as the water supply devices, the peristaltic pump at a fixed speed pushed the background solution (KCL solution concentrations of 0.01 mol/L, 0.05 mol/L and 0.1 mol/L) with adjusted pH (5, 6 and 7) from the bottom slowly into the soil column in order to drain the air in soil column. When the soil column was saturated and the steady flow field was formed, with the pulse input method, 1.0 pv CIP solu- tion of 50 mg/L was input from the upper soil column, and then the soil column was rinsed with KCl back- ground solution. The auto collector collected the flow fluid until the observed CIP concentration tended to zero in the flow fluid (two replicates for each experiment). By the way, pv = vt/l is a dimensionless time, where v represents the pore water velocity (cm/h), t represents time (h), and l represents the soil column length (cm).
2.4. Data Processing
2.4.1. Adsorption Amount
 (1)
(1)
where S is the CIP equilibrium adsorption on quartz sand (mg/kg), C0 is the CIP concentration in the added solution (mg/L), C is the CIP concentration in the balanced solution (mg/L), V is the equilibrium liquid volume (L), and m is the mass of added quartz sand (kg).
2.4.2. Fitting Equation
Empirical formulas of Langmuir and Freundlich isothermal adsorption curves can well fit the adsorption expe- rimental data. By fitting equations, each coefficient and certainty coefficient can be determined, and thus the CIP adsorption capacity on the quartz sand can be analyzed [19] .
Langmuir equation:
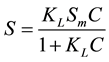 (2)
(2)
Freundlich equation:
 (3)
(3)
In the above equations, Sm represents the maximum CIP adsorption amount on the quartz sand (mg/kg), KL represents the affinity of the quartz sand to CIP adsorption, KF is a constant, and n is the adsorption reaction or- der (usually less than 1).
3. Results and Discussion
3.1. Static Adsorption
3.1.1. pH Influence on the Adsorption of CIP on Quartz Sand
As Figure 1 shows, in the pH range of 5.0 to 7.0, the higher pH leads to the lower CIP adsorption amount. pH generally affects the adsorption behaviors of adsorbent in water environment by changing the state of the soil surface and the morphology of solute molecules in solution. The adsorption on quartz sand mainly depends on its surface properties, and the surface reactivity mainly concerns about the surface hydroxyl groups. The CIP contains -NH3 and -COOH, which can combine with the H+ and OH− in solution respectively, so that the CIP can exist in solution in three forms including cation, zwitterion and anion. According to the pKa value of CIP (pKa1 = 6.10, pKa2 = 8.70) [20] , the proportion of CIP in different forms can be calculated as shown in Figure 2. When pH is less than pKa1, -NH3 in CIP can form CIPH+ by combining with H+ in solution, which benefits the CIP adsorption on the negatively-charged surface of quartz sand. In the pH range of 4 to 6, most of CIP is catio- nic. With pH increasing, the CIP cations in the solution decrease, while neutral ions gradually increase. When pH = 7.5, the CIP almost exists in electrically neutral zwitterionic form (+ - 0) [21] , and the CIP cationic groups can combine with negatively charged cations on the CIP surface by cation exchange. With the proportion de- cline of positive charge in CIP, the proportion of zwitterionic molecules gradually increases, so that the adsorp-
![]()
Figure 1. The adsorption isothermal of CIP at different pH in quartz sand.
![]()
Figure 2. Speciation of CIP at different pH [20] .
tion capacity of CIP on the quartz sand gradually weakens. Thus, the absorption of CIP on the quartz sand pro- ceeds mainly through cation exchange adsorption [22] . Meanwhile, the adsorption of CIP on quartz sand also relates to its own pH to some extent. Under acidic conditions, cation exchange effect is helpful to adsorb CIP on quartz sand, the CIP cations can combine with negatively charged quartz sand by cation exchange action; while under neutral conditions, the electrically neutral CIP molecules are absorbed on the quartz sand mainly by Van der Waals force.
3.1.2. Influence of Ionic Strength on the Adsorption of CIP on Quartz Sand
Figure 3 shows the comparative adsorption of CIP at different ionic strength (0.01 mol/L, 0.05 mol/L, 0.1 mol/L) when pH = 6. As can be seen, the bigger the ionic strength is, the lower the adsorption amount of CIP on quartz sand is. Probably, the increase of ionic strength makes the concentration of the competitive cation K+ on quartz sand increase, so that a large amount of negative charge points are occupied by K+ on quartz sand surface. Con- sequently, ions adsorbed by adsorption point tend to be saturated, which reduces the electrical adsorption ability of the quartz sand and thus the CIP adsorption amount on the quartz sand.
Table 1 shows the Kd value distribution coefficient of CIP in quartz sand at different ionic strength when pH = 6 (Kd is the ratio of CIP absorption amount per unit mass of soil to the CIP concentration in equilibrium solu- tion, L∙kg−1). Kd value can be used to describe the distribution of different cations between solid phase and liq- uid phase in soil. Low Kd value means that most CIP exists in the soil solution with potential activity and trans- port ability, while high Kd value indicates that there is a strong affinity between CIP and the surface of soil par- ticles so that CIP can be easily adsorbed by the surface of soil particles.
As shown in Table 1, Kd value on the quartz sand gradually decreases with the increase of CIP concentration in equilibration solution. Meanwhile, as ionic strength of the solution increases, Kd value of CIP on quartz sand decreases. Through the analysis above, the adsorption of CIP on quartz sand proceeds mainly by the ion ex- change between the negatively charged quartz sand surface and CIP cationic groups. With the increase of cation concentration in quartz sand solution, they compete with CIP cation active groups for active adsorption sites, so that the adsorption amount of CIP decreases. With the increase of initial CIP concentration, Kd value of CIP on quartz sand decreases, indicating the decrease of absorption rate in balanced solution. In low equilibrium con- centration, specific adsorption with high energy absorption sites dominates the adsorption process. With the in- crease of CIP concentration in balanced solution, specific adsorption sites are gradually occupied and non-spe- cific adsorption increases. As a result, the adsorption capacity of quartz sand relatively reduces, and Kd value of CIP decreases [23] -[24] .
3.1.3. Data Fitting for the CIP Adsorption on Quartz Sand
Figure 4 shows the fitting results of the CIP adsorption on the quartz sand by the Langmuir and Freundlich ad- sorption isothermal models. Obviously, the CIP adsorption characteristics on the quartz sand are nonlinear, and the obtained adsorption parameters are shown in Table 2. In the fitting process by Langmuir equation, the cor- relation coefficient R2 is larger than 0.806, while the correlation coefficient R2 is larger than 0.886 by Freundlich equation. Meanwhile, n is between 0.3 and 0.6, which shows that the adsorption isotherms are S-type adsorption
![]()
Figure 3. The adsorption isothermal of ciprofloxacin at different ionic strength in quartz sand.
![]()
Table 1. The distribution coefficient of CIP at different ionic strength in quartz sand when pH = 6.
![]()
Table 2. The adsorption isotherm parameters of CIP at different pH and ionic strength.
Note: R2 is the certainty coefficient, which represents the fitness of fitting values and measured values. The closer to 1 means the better fitness.
isotherms and that the adsorption type is nonlinear adsorption. Generally, this adsorption type can be attributed to the competition between water molecules and CIP molecules for adsorption sites on the soil surface. At low CIP concentrations, the affinity of CIP to the soil is weaker than that to the water phase; while with the in- crease of CIP concentration, its affinity to the soil increases, so that the adsorption amount increases. In contrast, if “n > 1”, the adsorption isotherm belongs to L-type adsorption isotherms. At low concentrations, the affinity of CIP to the soil is stronger than that to the water phase; while with the increase of CIP concentration, its affinity to the soil decreases, so that the adsorption weakens [25] . KF and KL can be used to evaluate the adsorption affinity of CIP on the quartz sand. As can be seen from Table 2, at a constant pH, the higher the ion intensity is, the smaller the affinity of CIP to the soil is, and thus the greater the fitted maximum adsorption amount is. The result is consistent with the experimental result. Under the same ionic strength, for Freundlich model, the higher the pH is, the smaller the affinity of CIP to the soil is, which is consistent with experimental result; while the adsorption isotherm fitting result by Langmuir model is not consistent with the experimental result. That is to say, Langmuir equation has certain limitations to fit the adsorption isotherm of competitive ions.
Adsorption reaction is the most important process during the transport and transformation of CIP in quartz sand, which can reflect the degree of interaction between CIP and the quartz sand and predict the stability of CIP in the quartz sand. Generally, the CIP is very stable if it can be strongly adsorbed by quartz sand, so that it is easier to accumulate in quartz sand instead of migration. In contrast, if the CIP cannot be absorbed and fixed easily on quartz sand, it is easy to be transported into surface water or groundwater under eluviations effect. The above results show that low pH and low ionic strength are conductive to the fixing of CIP in the soil, which eliminate its migration with the water.
3.2. Soil Column Transport Experiments
3.2.1. The Breakthrough Curves of CIP Transport in Quartz Sand at Different pH
Figure 5 shows the breakthrough curves of CIP in quartz sand at different pH (5, 6, 7) when the ionic strength is 0.05 mol/L. The CIP solution of pH = 7 starts to outflow when pv = 0.47, then the CIP solution of pH = 6 starts to outflow when pv = 0.51, and finally the CIP solution of pH = 5 starts to outflow when pv = 0.54. The greater the pH is, the sooner the outflow is. The reason is that the greater pH usually weakens the CIP adsorption capac- ity on quartz sand, so that the CIP can outflow very fast, with a stronger migration. Prior to 1.0 pv, it is the ad- sorption stage, followed by the desorption stage. When pv = 1.0, the backwash stage (adsorption-desorption phase) starts, and then the CIP concentration gradually increases. When pv = 1.63, the solution of pH = 5 reaches the maximum concentration, with a relative concentration C/C0 of 0.88; when pv = 1.56, the solution of pH = 6 reaches the maximum concentration, with a relative concentration C/C0 of 0.96; when pv = 1.65, the solution of pH = 7 reaches the maximum concentration, with a relative concentration C/C0 of 0.89. After that, the CIP con- centration decreases rapidly. During this process, the solution of pH = 7 desorbs most rapidly, followed by the solution of pH = 6 and the solution of pH = 5. The higher the pH is, the smaller the adsorption amount is, and thus the greater the desorption amount is. In the transport process from solution outflow to the concentration be- ing zero, the volume of the solution of pH = 5 is 2.50 pv, that of pH = 6 is 2.48 pv, and that of pH = 7 is 2.36 pv. The CIP starts to outflow earliest when pH = 7, followed by pH = 6 and pH = 5. This difference may be related to the different adsorption capacity of CIP at different pH. The lower the pH is, the stronger the adsorption ca- pacity is, and thus the longer the outflow time is. On the contrary, the greater the pH is, the shorter the outflow
![]()
Figure 5. The breakthrough curves at different pH when the ionic strength is 0.05 mol/L.
time is. When pH = 5, the desorption phase has a long tail due to the slow desorption caused by large adsorption amount. Hence, if the soil is in alkaline environment, the CIP concentration in the soil will increase, and the CIP is easier to migrate in the soil, thus increasing the risk of groundwater contamination.
3.2.2. The Breakthrough Curves of CIP Transport in the Quartz Sand at Different Ionic Strength
Figure 6 shows the breakthrough curves of CIP in quartz sand at different ionic strength (0.01, 0.05, 0.1 mol/L) when pH = 6. In the adsorption phase, the CIP adsorbs fast and outflows slowly at the ionic strength of 0.01 mol/L. The greater the ionic strength is, the faster the outflow is. The reason may be that a large number of K+ ions adsorbed on the quartz solid surface compete with CIP for the adsorption sites, resulting in the decrease of CIP adsorption amount on quartz sand and further delaying the outflow. In the desorption stage, at the ionic strength of 0.01 mol/L, CIP desorbs slowly, and there presents a tail. This process is a continuous and slow de- sorption process. With the ionic strength increasing, the desorption becomes fast, which promotes the transport of CIP in quartz sand. At the same time, when the ionic strength is large, a large number of K+ ions occupy the negatively charged adsorption sites on the surface of medium, and the negatively charged adsorption sites tend to be saturated, which reduces the adsorption of CIP. In addition, the increase of ionic strength will enhance the ionic interaction in systems and reduce the ion activity coefficient, which decreases the effective concentration of CIP and accelerates its transport in the soil column. Hence, if the soil is contaminated by a high-salt waste- water, it will accelerate the desorption of CIP and promote its transport in the soil, thus polluting the groundwa- ter.
4. Conclusions
In summary, the CIP adsorption and transport characteristics in quartz sand were investigated at different pH and ionic strength conditions, and some conclusions can be drawn as follows:
1) The adsorption of CIP on the quartz sand belonged to cation exchange adsorption, and the variations of pH and ion morphology had a significant influence on the adsorption capacity.
2) When pH was in the range from 5 to 7, the higher the pH was, the lower the adsorption capacity of CIP on quartz sand was; the greater the ionic strength was, the lower the CIP adsorption amount on quartz sand was. That was to say, the low pH and low ionic strength were in favor of CIP adsorption on quartz sand. With the in- crease of CIP concentration in balanced solution, the Kd value of CIP in quartz sand decreased; meanwhile, with the increase of ionic strength in the solution, the Kd value of CIP in quartz sand decreased.
3) The CIP adsorption on quartz sand could be described by the Langmuir and Freundlich equations, and the Freundlich equation fitted the adsorption isotherms better.
4) When pH was in the range from 5 to 7, the higher the pH was, the faster the transport of the CIP on quartz sand was, and thus the earlier the outflow was. That was to say, the higher the pH was, the stronger the adsorption capacity was, and thus the shorter the outflow time was. Meanwhile, the increase of ionic strength would
![]()
Figure 6. The breakthrough curves at different ionic strength when pH = 6.
reduce the absorption of CIP on quartz sand, which led to the faster transport and the earlier outflow. As a large number of K+ ions occupied the negatively charged adsorption sites, the medium surface tended to be saturated, so that the adsorption capacity of CIP on quartz sand was reduced.
Acknowledgements
This study was accomplished with participation of the undergraduates innovative experimental group members Jiang-Long He, Yu Li, Zhao-Xi Fang, Xin Li and Ya-Kun Hu.
Foundation Item
National Natural Science Foundation of China (40771095), Qingdao people’s livelihood project (13-1-3-132-nsh).
NOTES
*The first author: Xiujiao Xu (1988), Female, Hebei, Master, is mainly engaged in the numerical simulation of underground water flow and solute transport in the environment.
#Corresponding author.