1. Introduction
In the last few decades, the semiconductor zinc oxide (ZnO) with a wide band gap energy (3.37 eV) at room temperature and high exciton binding energy (60 meV) [1] , has been used as host material for the doping of rare-eart (RE) and transition metals (TM) ions, which exhibit optical and magnetic activity [2] - [6] . The RE- doped ZnO nanocrystals have an high potential to be used in integrated optoelectronic devices such as infrared and visible (blue, green, red) luminescent devices because they present a highly efficient luminescence even at room temperature [7] -[11] ; the emission process is determined by the internal dynamics of the RE3+ electronics transitions governed by the relative energy of the 4f emitting level including the direct 4f-4f and indirect process 5D0 → 7fi with i = 0 - 4. From all the RE3+ ions, Eu3+ ion is the most representative and most widely studied and actually continue being used as dopant in many host compounds. Its color emission characteristic is the red color which is used in the fabrication of light emitting devises. Actually, it is possible to obtain and dope by several methods of the ZnO semiconductor in all your existent nanostructures: powders, nanowires, nanorods, nanobelts, nanonedles, nanorings, and nanoflowers [12] - [17] . Such methods are: radio frequency magnetron sputtering [4] , spray pyrolysis deposition [18] , hydrothermal synthesis [19] , sol gel technique [20] , thermal evaporation reac- tive [21] , chemical vapor deposition [22] , reactive magnetron sputtering [23] , and solution combustion method [24] . The last method is considered as fast, simple, easy controlabilited, low cost and great scale production. In this work we synthesized ZnO intrinsic and Eu3+ doped ZnO (ZnO/Eu3+) by a solution combustion method as a function of the Eu3+ ion concentration in wt% and after annealing at 900˚C by 24 h, also, the photolumines- cence emission intensity of the ZnO:Eu3+ as a function of the Eu3+ ion concentration is fited empirically by a exponential function and a phenomenological description and interpretation of the observed data is given.
2. Experimental
Undoped and Eu3+ ion doped ZnO nanocrystals ZnO:Eu3+ were prepared by solution-combustion synthesis method as a function of the Eu3+ ion concentration in wt% using as source materials Zinc nitrate hexahydrated (Zn(NO3)2∙6H2O) as oxidizer, urea (H2NCONH2) as fuel, and Europium chloride (EuCl3) as dopant. In this process the follow redox chemical reaction stoichiometric oxidizer-fuel producing ZnO, H2O vapor, CO2, and N2 is obtained:
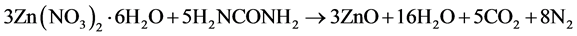 (1)
(1)
The equation (1) is obtained by taken in account the oxidizer/fuel molar radio (O/F = 1) required for a stoichiometric reactive solution which is determined by summing the total oxidicing and reducing valencies in the oxidizer compound and dividing it by the sum of the total oxidation and reducing valencies in the fuel compound [25] . Accordingly, for the complete combustion of Zinc nitrate-urea reactive solution, the molar ratio becomes 5/3, the molar balanced equation (1) was obtained with this value. Using the atomic weight concept, the equation (1) was translated to grams/mol and used to obtain 3 gr of ZnO powder for all the 0, 1, 3, 5, 7, and 10 wt% Eu3+ ion concentrations with respect to 3 gr of ZnO. From the translated grams/mol equation (1) (no shown), 8.31 gr of Zn(NO3))2∙6H2O, 3.68 gr of H2NCONH2 plus the numerous of grams corresponding to each Eu3+ ion concentration were mixing with 20 ml of H2O and stirring vigourosly in a flask glas and after put on a hot plate at 500˚C, after a few minutes the reactive solution boils, foams, ignites, and burns with a incandescent flame at a approximate temperature of 1200˚C [26] producing 3 gr of ZnO powders approximately. After all the samples are annealing at 900˚C by 20 h. The ZnO:Eu3+ samples thus obtained were structurally characterized by X-ray diffraction (XRD) technique using a Philips PW 1800 diffractometer with Cu kα radiation (1.5406 Å), XPS method was used to verify the Zn-O, Eu-O and O1s bonding energies (BE), the photoluminescence (PL) of the ZnO:Eu3+ samples was studied by means of a spectrofluorometer FluoroMax-P that uses a xenon lamp as excitation source, The wavelength excitation was of 270 nm, and finally, the morphology of the ZnO:Eu3+ powders was recorded using a scanning electron microscopy SEM) JEOL JSM 840 A.
3. Results and discussion
3.1. Structural characterization
figure 1 shows the X-ray diffractograms of the ZnO:Eu3+ powders as a function of the Eu3+ ion concentration and annealing at 900˚C by 20 h. From the XRD patterns, can be observed that all the diffraction peaks can be indexed to the majority phase hexagonal wurtzite tipe ZnO structure for all samples (JCPDS card #89-(102), moreover, for all the Eu3+ ion concentrations a little peak at 2θ = 28.4˚ is observed, which is attributed to the (210) plane of the Eu2O3 minority phase (JPDS card #86-2476), Park et al. [27] reported diffraction peaks due to Eu2O3 after annealing the Eu3+ doped ZnO at temperatures higher 1000˚C in air and vacun conditions, no dif- fraction peaks were detected from other impurities. However, the intensity of the (101) peaks decrease with the
![]()
Figure 1. XRD patterns of the ZnO/Eu3+ samples as a function of the Eu3+ ion concentration in wt%.
increase in the Eu3+ concentration which create some disorder in the ZnO structure. This is further verify by the increase of he FWHM of the (101) peak with the Eu3+ concentration indicating a decrease in the crystallite size; effectively, using the Scherrer formula, the average crystallite size calculated from characteristic peak (101) was 41 nm for the ZnO intrinsic and decreased to 18 nm for the ZnO/Eu3+ samples doped with 10 wt% of Eu3+. In addition, in figure 2, are showed the diffraction patterns of the characteristic peaks (100), (002) and (101) of the doped ZnO/Eu3+ samples as a function of the Eu3+ ion concentration, it can be observed that the three peaks shifted towards a bigger 2θ vaue for 1, 3, and 5 wt% of Eu3+ concentration compared with the ZnO intrinsic, and further this peaks returned to the same position what that of the ZnO intrinsic, this change in the diffraction peaks towards a bigger 2θ value show a decrease of the lattice parameter and cell volume, this result is contrary to the results founded by another researchers [4] [28] [29] since the doping of the bigger size Eu3+ ion (effective ionic radio ri = 9.74 nm) compared to that of the smaller Zn2+ ion (ri = 7.40 nm) and therefore its incorporation in the ZnO lattice must lead to a increase in the cell parameters and volume, it is due to a low solubility of the Eu3+ ion in the ZnO lattice even for 1 wt% Eu3+ concentration, Yang et al. reported that the solution limit in the Eu3+ doped is below 0.2 wt% Eu3+ ion [10] , this low solubility increase the yield of Eu2O3 species with increase of Eu3+ concentration at the ZnO nanocrystals surfaces [30] as is showed in the XRD diffraction patterns and at the SEM images showed in figure 4.
3.2. XPS analysis
To check the Eu3+ presence in the ZnO host, and its effect as a doping agent, on the surface chemical composi- tion, XPS whole spectra of the ZnO:Eu3+ samples was obtained as a function of the Eu3+ ion concentration, figure 3 shows the binding energy (BE) for each ZnO:Eu3+ samples doped with the Eu3+ ion at 0, 1, 3, 5, 7, and 10 wt%, focusing in particular on the binding energies of the typical lines of O, Zn, and Eu. The O1s photoelec- tron peak showed a BE = 530.1 eV [31] [32] , attributed to the lattice oxygen in a Zn-O-Zn network. With re- spect to the Zn ion presence the core-level photoelectron peaks showed a BE = 1021.5 eV corresponding to the core-level Zn2p3/2 revealing the presence of Zn2+ ions in an oxide environment, the before analysis include to both undoped and Eu3+ ZnO doped. For all the Eu3+ ion concentrations, in your XPS spectrum respective there are two peaks in the Eu3+3d region (1110 - 1170 eV) with BE = 1134.5 eV and 1164.3 eV corresponding to Eu3+3d5/2 and Eu3+3d3/2 respectively; both peaks are due to multiple spin-orbit interactions and are consistent with the reported values for Europium-coordinated ions which indicates that the oxidation states of Europium ions are trivalents for the ZnO:Eu3+ samples [29] [33] . Non other satelite peak can be seen on the XPS spectra.
3.3. Morphology of the ZnO/Eu3+ samples
figure 4 shows the SEM images of the as prepared ZnO:xEu3+ (x = 0, 1, 3, 5, 7, and 10 wt%) samples as a
![]()
Figure 2. XRD patterns of the (100), (002) and (101) peaks of the ZnO; Eu3+ samples as a Function of the Eu3+ ion concentration in wt%.
![]()
Figure 3. XPS spectra of the ZnO/Eu3+ samples as a fuction of Eu3+ concen- tration.
function of the Eu3+ ion concentration in wt% and after annealing at 900˚C by 24 h. As can be observed, there are not clear morphological differences between undoped and Eu3+ doped samples indicating that the Eu3+ dopant in the ZnO host do not affect its morphology of the ZnO:Eu3+ nanocrystals. The grains of the ZnO have a mean large about 0.6 µm. Moreover, it can be seen that Eu3+ crystallites are adhered to the ZnO surface increas- ing with the Eu3+ ion concentration.
3.4. Photoluminescence properties
figure 5 shows the room temperature photoluminescence spectra (PL) of the ZnO:Eu3+ nanocrystals as a
![]()
Figure 5. Room temperature photoluminescence spectra of the ZnO:Eu3+ samples as a function of the Eu3+ ion concentration irradiated with a excita- tion wavelength of 270 nm.
function of the Eu3+ ion concentration in wt% and after annealing at 900˚C by 24 h, the samples were irradiated with an excitation wavelength of 270 nm (in the UV range). It is observed that the ZnO:Eu3+ samples doped with 1, 3, 5, 7 and 10 wt% are all alike: they present a green broad emission band from 400 nm to 600 nm centered about 516 nm, this band is due to the intrinsic defects emission of ZnO host. however, in addition to the broad band characteristic of defects in ZnO appear the photoluminescence spectra of the Eu3+ doped powders: effec- tively, the sharp peaks in 579, 591, 613, 618, 650 and 770 nm are related to the direct intra-4f transitions in Eu3+ ions 5D0 → 7Fj (j = 0, 1, 2, 3, 4), the most intense emission is associated to the 5D0 → 7F2 emission in the red spectral region (613 nm) and is due to an allowed electric-dipole transitions with inversion antisymmetry [19] , which results in a large transition probability in the crystal field; in our case this emission is split in two compo- nents of 613 and 618 nm, theoretically, the 7F2 level gives three crystal field levels of A1 and 2E with 3Cv sym- metry, because A1 and one of two E levels have close energy levels, two emission peaks (A1 and E at 613 and 618 nm) can be overlapped in the PL spectra [34] [35] . The peak at 591 nm is due to the 5D0 → 7F1 transition, is an allowed magnetic-dipole transition, if the Eu3+ ion is situated in a symmetry center in the ZnO matrix, elec- tric-dipole transitions between the 4f6 levels are strictly forbidden by the Laporte selection rule (equal parity) while the magnetic-dipole is allowed. Thus, the intensity ratio of 5D0 → 7F2 to 5D0 → 7F1 transition known as symmetry ratio can provide information about the quality structural of the material [35] [36] . The symmetry ra- tio in our material about 8 indicating low inversion symmetry when the Eu3+ ion is incorporated into hexagonal ZnO host by substitution on Zn sublattice. The peak at 579 nm is due to the forbidden 5D0 → 7F0 transition due to the same total angular momentum, indicating that some Eu3+ ions possibly occupy other sites as interticial sites [37] [38] . Figure 6 shows the integrated emission intensity variation of the 5Do → 7F2 transition as a func- tion of the Eu3+ ion concentration, the empiric graphic was fited, to the exponential function I(%) = 1.3E6*e0.23% where % is the Eu3+ ions concentration, the solid curve in figure 5 represents the fit to the experimental data, the integrated intensity increases as an exponential function as the doping concentration increase which indicates the enhanced energy transfer between the ZnO host and activator Eu3+ ions. The concentration Quenchin effects do not appear in this concentration range.
4. Conclusion
In this work undoped and Eu3+ doped ZnO nanocrystals were synthesized by a solution combustion technique. From XRD studies, the ZnO:Eu3+ structure resulted in the hexagonal wurtzite ZnO structure for all the Eu3+ ion concentrations; and also showed the existence of the Eu2O3 phase mixed with the ZnO phase. The effect of Eu3+ doping percentage on the nanocrystals showed that with increasing doping percentage, disorder in the nanocrys- tals increases. From the XPS spectra, the oxidation states of the Eu ions are trivalent for the Eu3+ doped ZnO nanocrystals. Solution combustion synthesized Eu3+ doped ZnO nanocrystals are found to emit red and green
![]()
Figure 6. Fit of the intensity emission integrated of the transition 5D0 → 7F2 as a funtcion of the Eu3+ ion concentration in wt%.
light. The intensity of the emission was related to doping concentration by means of an exponential fit.
Acknowledgements
The authors wish to tank Adriana Tejeda (IIM) for his support in carrying out X-ray measurements; Omar Novelo (IIM) for their support in the scanning electron microscopy, to M. Canseco Martínez by his support in chemical reactions.