Mathematical Modelling of Sterile Insect Technology for Mosquito Control ()
1. Introduction
Malaria is a major life-threatening vector-borne disease transmitted through mosquitoes. The disease got its name from bad air (malaria) as it was thought that the disease came from fetid marshes. Later in 1880, it was discovered that the real cause of malaria was Plasmodium [1] a single cell parasite which can only be transmitted from one person to another by the bite of female Anopheles mosquito. The male Anopheles mosquitoes are not involved in disease transmission as they don’t require blood to nurture eggs as their female counterparts do [2] .
The disease is more prevalent in the tropical and sub-tropical regions of the world and causes more than 300 million acute illnesses and at least one million deaths annually [2] . People living in the world’s poorest countries with a total population of approximately 40% of the world’s population, are at a high risk of the malaria ailment. According to the World Malaria Report by World Health Organization (2005), 90% of deaths caused by malaria take place in Africa, primarily among young children, pregnant women and their unborn children. A child in Africa dies every 30 seconds because of malaria and those who survive the severe episode of malaria might suffer from learning impairments or brain damage. There are no available vaccines yet to prevent infection by the malaria virus and the avoidance of mosquito bites is the most effective protective measures against infection.
In vector borne disease control strategy, it is important to include the mating behavior of the mosquito, which is an aspect of mosquito biology that is not fully understood [3] . The buzz of a flying female mosquito acts as a mating signal for attracting males [4] . Mate assessment interactions in swarming insects occur when these insects enter swarms and this happens very quickly in flight [3] . In mosquito mating swarms, it is important to converge quickly to a mate harmonic signal before others; this is to ensure that a desirable mate locks faster into its signal, than the signal from a swarming competitor [5] . This is an indication that harmonic convergence may be used in mate assessment. The female mosquito has the ability to react to variation in flight tone, which further suggests that this signal may be used to assess the males during precopulatory interactions [6] . Single females fly into the swarm and are detected by their lower wing-beat frequency [7] [8] . It has been reported that several males may arrive near the female, and the female departs with one of the males from the swarm in copula. Also, larger males were more successful in mating than smaller ones [9] [10] . Thus there is evidence that sexual selection operates when these insects enter the swarms, and hence should be incorporated into any reasonable model.
The Sterile Insect Technology has been tried in a number of scenarios, as an attempt to combat malaria and dengue with little success. Many of the earlier approaches were based on ordinary differential equations. They made several assumptions such as 1:1 sex ratio [11] , addressing fraction of the population as either infected or uninfected and many more. [12] proposed two mathematical model and proved theoretically and computationally that the control of wild female mosquitoes is effective provided that the number of sterile male mosquitoes released is above the threshold number; the study also exposes the fact that the success of SIT depends on the entomological parameters of the wild anopheles mosquito as well as on parameters released to the sterile males. In this study, we observed the effectiveness of the Sterile Insect Technology for Aedes aegypti mosquitoes when the female sexual preference for wild males over sterile males is incorporated and the computer simulation result shows that if sexual selection is incorporated into a reaction diffusion system, modelling the spread of Aedes aegypti mosquitoes, the sterile insect technology can still be a successful control measure if the injection of sterile males is large enough for a sufficient period.
The objective of this study was to develop a computer program for solving the model equations developed by [13] , the dynamics of a PDE model for Aedes aegypti mosquito incorporating female sexual preference and computer simulation to estimate the effectiveness of the Sterile Insect Technology for Aedes aegypti mosquitoes when the female sexual preference for wild males over sterile males is incorporated.
2. Methodology
2.1. Model for Control of Wild Mosquito Population via the Sterile Insect Technology
PDE model for Aedes aegypti mosquito incorporating female sexual preference is proposed by Parshad and Agusto (2011).
This model is an extension of the model in [14] to include both spatial spread, and preferential selection of the female mosquito for the wild males, against modified sterile males. The mosquito population is divided into male and female classes. The female classes are further divided into immature and adult depending on the insect sexual preference.
The per capita mating rates of an unmating female with a wild male mosquito are given by:
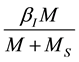 (1)
(1)
and 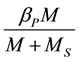 (2)
(2)
where: 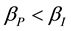
The per capita mating rate of a female with a sterile male is given by:
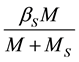 (3)
(3)
where: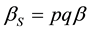 and
and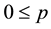 ;
; 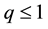
The model is described in Equation (4) and the description of the variables and parameters of the model are shown in Table 1
 (4)
(4)
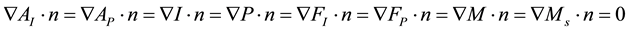
On  we also impose suitable initial conditions
we also impose suitable initial conditions
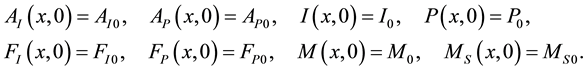
Assumptions made by Parshad and Agusto (2011):
・ A female mosquito mates once in its life, and oviposit its eggs in different places during its entire life [15] .
・ The population of females with sexual preference is maintained by mutation from female without sexual preference at the rate  since the mating rate of the wild females with sexual preference is lower than mating rate of females without sexual preference.
since the mating rate of the wild females with sexual preference is lower than mating rate of females without sexual preference.
・ The females with preference do not mate with the sterile male (even in small probability). This is to incorporate selection in the model, and helps to clearly differentiate between the classes I and P. Note, there is no conclusive evidence, that sexual selection exists in mosquito mating, however there are a number of studies, [3] [6] -[9] that suggest, this might be so.
・ We assume what is known as a mutation-selection balance, [4] . Essentially, we assume that females that display preference in mate selection have a lower mating rate overall (e.g. due to time wasted searching for a male). In the absence of a high number of fit males, these females are at a fitness disadvantage, because they do not reproduce as quickly. As a result, over time, they would be driven to extinction by competitive exclusion with the females without preference. Thus preference can be thought of as a deleterious allele. In many population biology models, deleterious alleles are assumed maintained in the population by mutation. The deleterious alleles keep appearing as a result of mutation, but because they are selected against, they are only maintained at low levels.
2.2. Model Simulation
In simulating, model Equation (4), the parameter values in Table 2 and the assumed initial values in Table 3 were used. The computer program and algorithm for the simulation is shown in appendix 1 and 2 while the flowchart is shown in Figure 1.
 ability of dispersion,
ability of dispersion, 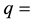 percentage of reduction of mating capacity and
percentage of reduction of mating capacity and 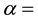 release rate of sterile male mosquitoes Values of
release rate of sterile male mosquitoes Values of  are sensitive to the environment into which the genetic male mosquito is being released [18] [19] .
are sensitive to the environment into which the genetic male mosquito is being released [18] [19] .
![]()
Table 1. Description of the variables and parameters of the model.
Courtesy: [13] .
![]()
Table 2. Parameter values used for simulation study [16] [17] .
![]()
Table 3. Assumed Initial values used for simulation.
3. Results
The computer program for the simulation is presented in Appendix 1.
In the simulation presented on Figures 2-5, we used model Equation (4), the parameter values in Table 2 and assumed initial values as shown in Table 3. The model was solved using MATLAB assuming constant initial values while varying the initial value of released sterile male mosquitoes. For the population control, the ratio of the number of released sterile males to the number of wild males in the local population was 10:1.
Figure 2(a) shows the wild mosquito population dynamics while Figure 2(b) shows the sterile male mosquito population dynamics when the initial released sterile male was 4000. Figure 3(a) and Figure 3(b) show the population dynamics for both the wild mosquito and sterile male when the initial released sterile male was 6000. Figure 4(a) and Figure 4(b) show the population dynamics for both the wild mosquito and sterile male when the initial released sterile male was 50,000 and Figure 5(a) and Figure 5(b) show the population dynamics for both the wild mosquito and sterile male when the initial released sterile male was 100,000.
![]() (a)
(a)![]() (b)
(b)
Figure 2. (a) Dynamics conditions of population under SIT control (Ms = 4000). (b) Dynamics conditions of population under SIT control (Ms = 4000).
![]() (a)
(a)![]() (b)
(b)
Figure 3. (a) Dynamics conditions of population under SIT control (Ms = 6000). (b) Dynamics conditions of population under SIT control (Ms = 6000).
4. Discussion of Results
The sterile insect technique (SIT) is a biological control which disorders the natural reproductive process of insects; male insects are first made sterile by gamma radiation before releasing them in large numbers into the environment to mate with the native wild insects. Insect populations can be controlled by the release of large numbers of sterile males. Thus, if a female mate with a male that has no sperm or whose sperm was rendered unviable, this female will have fewer or no offspring. When many sterile males are released, the local population tends to decline or become wiped out. For population control, the crucial parameter is the ratio of the number of released sterile males to the number of males in the local population, which ideally should be around 10:1 [20] .
As demonstrated on Figure 4(a) and Figure 4(b) for sterile male mosquito released below 40,000, the wild mosquito population decreases while the sterile male mosquito population increases. For mosquitoes, male-only release is considered essential since sterile females will bite and so may transmit disease, whereas male mosque-
![]() (a)
(a)![]() (b)
(b)
Figure 4. (a) Dynamics conditions of population under SIT control (Ms = 50,000). (b) Dynamics conditions of population under SIT control (Ms = 50,000).
![]() (a)
(a)![]() (b)
(b)
Figure 5. (a) Dynamics conditions of population under SIT control (Ms = 100,000). (b) Dynamics conditions of population under SIT control (Ms = 100,000).
toes do not bite. These released insects compete for mates with wild males; a wild female mating with a released sterile male has no or fewer progeny, so the population tends to decline. This means that the sterile male mosquitoes have dominated the environment. Hence if the mosquito population has not grown yet sufficiently or it has been reduced by some other measure e.g. destroying the breeding places, it can be controlled by release of sterile males.
As shown in Figure 4(a) and Figure 4(b) for sterile male mosquito released above 40,000, the wild mosquito population decreases and the sterile male mosquito population decreases as well. In SIT, some or all of the progeny of released individuals die as a consequence of inheriting one or more dominant lethal mutations, so the population tends to decline. For population control purposes, the only timing requirement for the lethal system is that death occurs before reproductive maturity.
This implies that if sufficient sterile male mosquitoes are released for a sufficient period, the wild mosquito population decreases to zero and so the environment is dominated by the sterile male mosquitoes and with time these sterile male mosquitoes start dying since there is no reproduction. Therefore, if the injection of sterile male mosquitoes is large enough the environment will be eradicated completely of mosquitoes over time.
Essentially our results show that if sexual selection is incorporated into a reaction diffusion system, modeling the spread of Aedes aegypti mosquitoes, the sterile insect technique can still be a successful control measure, if the injection of sterile males is large enough.
5. Conclusions
From the simulation, it was revealed that the success of SIT depends on the parameters of the wild mosquitoes as well as on parameters related to the sterile male mosquitoes.
We established that for a released value of the sterile male mosquito below 40,000, there was reduction in the wild mosquito population and domination of the sterile male mosquitoes in the environment. For a released value of the sterile male mosquito above 40,000, there was eradication of mosquitoes in the environment over time as the sterile male mosquito population decreases. The result shows that if sexual selection is incorporated into a reaction diffusion system, modelling the spread of Aedes aegypti mosquitoes, the sterile insect technology can still be a successful control measure if the injection of sterile males is large enough. Therefore, if sufficient sterile insects are released for a sufficient period, the target population will be controlled or even locally eradicated.
Acknowledgements
This project was supported by Lagos State Government (Local Scholarship Award). The authors would like to thank Mogbojuri Babatunde (Lead software developer/system engineer) at Global Accelerex Ltd. who was there in the course of this project to provide answers to every professional question.
Notations
![]() for the adult form, mating singles without sexual preference
for the adult form, mating singles without sexual preference
![]() for the adult form, mating singles with sexual preference
for the adult form, mating singles with sexual preference
![]() immature phase of the mosquitoes (eggs, larvae and pupae) from adults without sexual preference
immature phase of the mosquitoes (eggs, larvae and pupae) from adults without sexual preference
![]() immature phase from adults with sexual preference
immature phase from adults with sexual preference
![]() mating fertilized females without sexual preference
mating fertilized females without sexual preference
![]() mating fertilized females with sexual preference
mating fertilized females with sexual preference
![]() wild male mosquito population
wild male mosquito population
![]() sterile insect (sterile male mosquitoes)
sterile insect (sterile male mosquitoes)
![]() oviposition rate per female mosquito which is proportional to female density, but it is also regulated by a carrying capacity effect, C, which is related to the amount of available nutrients and space
oviposition rate per female mosquito which is proportional to female density, but it is also regulated by a carrying capacity effect, C, which is related to the amount of available nutrients and space
![]() the rate at which the aquatic population becomes winged mosquitoes
the rate at which the aquatic population becomes winged mosquitoes
![]() proportion that transforms into female
proportion that transforms into female
![]() proportion that transforms into male
proportion that transforms into male
![]() mortality rates of the immature form with and without sexual preference
mortality rates of the immature form with and without sexual preference
![]() mortality rates of the fertilized female with and without sexual preference
mortality rates of the fertilized female with and without sexual preference
![]() mortality rates of the unmating female with and without sexual preference
mortality rates of the unmating female with and without sexual preference
![]() mortality rates of the wild males
mortality rates of the wild males
![]() mortality rates of the sterile males
mortality rates of the sterile males
![]() the rate at which sterile males are released and sprayed
the rate at which sterile males are released and sprayed
![]() the effectiveness of sterile male introduction regarded to the spatial distribution of female insects
the effectiveness of sterile male introduction regarded to the spatial distribution of female insects
![]() physiological modifications induced by the sterilization technique
physiological modifications induced by the sterilization technique
Appendix 1
The computer Program for the simulation of a PDE model developed by Parshad and Agusto 2011 for Aedes aegypti mosquito using SIT when the female sexual preference is incorporated. Function mc = mosquito control![]() n = 8; % n represents the number of equations or variables
n = 8; % n represents the number of equations or variables![]() ;
;
Part A
This section of the code declares all constants
![]() ; % mortality rates of the immature form with and without sexual preference
; % mortality rates of the immature form with and without sexual preference
mu = 0.5; % mortality rates of the unmating female with and without sexual preference
muf = 0.5; % mortality rates of the fertilized female with and without sexual preference
mum = 0.1; % mortality rates of the wild males
mums = 0.1; % mortality rates of the sterile males
Bi = 1.0;
del = 0.12;
Bp = 1.0;
Bs = 1.0;
phi = 50; % oviposition rate per female mosquito
gamma = 0.1; % the rate at which the aquatic population becomes winged mosquitoes
r = 0.5; % proportion that transforms into female
C = 600; % carrying capacity effect
alpha = 4000; %rate at which sterile males are released and sprayed
This section of the code solves the system of PDE’s
% the following definitions apply
![]() -Immature phase of the mosquitoes
-Immature phase of the mosquitoes
![]() -immature [phase from adults with sexual preference
-immature [phase from adults with sexual preference
![]() -for the adult form, mating singles without sexual preference
-for the adult form, mating singles without sexual preference
![]() -for the adult form, mating singles with sexual preference
-for the adult form, mating singles with sexual preference
![]() -mating fertilized females without sexual preference
-mating fertilized females without sexual preference
![]() -mating fertilized females with sexual preference
-mating fertilized females with sexual preference
![]() -wild male mosquito population
-wild male mosquito population
![]() -sterile insect (sterile male mosquitoes)
-sterile insect (sterile male mosquitoes)
![]() ;
;
![]() ;
;
![]() ;
;
![]() ;
;
![]() ;
;
![]() ;
;
![]() ;
;
![]() ;
;
Part B
This section allows user to specify initial value of variables for the system of PDE’s
Ai0 = input (“Enter a value for Ai (0) = ”);
Ap0 = input (“Enter a value for Ap (0) = ”);
I0 = input (“Enter a value for I (0) = ”);
P0 = input (“Enter a value for P (0) = ”);
Fi0 = input (“Enter a value for Fi (0) = ”);
Fp0 = input (“Enter a value for Fp (0) = ”);
M0 = input (“Enter a value for M (0) = ”);
Ms0 = input (“Enter a value for Ms(0) = ”);
This section solves the system of PDE’s
![]() ;
;
![]() ode45(@mosquitocontrol, [0 100], x0)
ode45(@mosquitocontrol, [0 100], x0)
This section generates the graph of the system of PDE's
Plot (t,x(:,1),t,x(:,2),t,x(:,3),t,x(:,4),t,x(:,5),t,x(:,6),t,x(:,7),t,x(:,8));
xlabel ('time (days)');
ylabel (“population”);
legend ('Ai', 'Ap', 'I', 'P', 'Fi', 'Fp', 'M', 'Ms')
title (“Graph showing population distribution of mosquitoes”);
figure;
plot (t,x(:,1),t,x(:,2),t,x(:,3),t,x(:,4),t,x(:,5),t,x(:,6),t,x(:,7));
xlabel (“time (days)”);
ylabel (“population”);
legend ('Ai','Ap','I','P','Fi','Fp','M')
title (“Graph showing population distribution of mosquitoes”);
figure;
plot (t,x(:,8));
xlabel (“time (days)”);
ylabel (“population”);
legend (“Ms”)
figure;
plot (t,x(:,7));
xlabel (“time (days)”);
ylabel (“population”);
legend (“M”)
figure;
plot (t,x(:,6));
xlabel (“time (days)”);
ylabel (“population”);
legend (“Fp”)
figure;
plot (t,x(:,5));
xlabel (“time (days)”);
ylabel (“population”);
legend (“Fi”)
figure;
plot (t,x(:,4));
xlabel (“time (days)”);
ylabel (“population”);
legend (“P”)
figure;
plot (t,x(:,3));
xlabel (“time (days)”);
ylabel (“population”);
legend (“I”)
figure;
plot (t,x(:,2));
xlabel (“time (days)”);
ylabel (“population”);
legend (“Ap”)
figure;
plot (t,x(:,1));
xlabel (“time (days)”);
ylabel (“population”);
legend (“Ai”)
Appendix 2
Algorithm for Mosquito control model
1) Define model parameters and variables
2) Input values of constants![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]()
3) Specify model equation
4) Input initial values of variables![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]()
5) Determine solution method for system of equations (4th order Runge-Kutta method)
6) Specify step increment and simulation range for independent variable (time)
7) Solve model equations using solution method specified above
8) Dimension array and align to grid
9) Start iterations
10) Compute variables at each point
11) Continue loop until last point
12) Print result
13) Plot result and print graphs
14) End simulation