Using Graphical and Multivariate Statistical Methods for Geochemical Assessment of Groundwater Quality in Oumé Department (Côte d’Ivoire) ()
1. Introduction
Any civilised society should consider the provision of safe drinking water a priority. This is so because safe drinking water is a basic need to human development, health and well-being. Chemically contaminated drinking water has been linked with a great majority of health problems. Chemical contaminants of drinking water such as nitrate, nitrite and N-nitrosamines are potentially harmful to man [1] [2] . Besides the health aspect of contaminated drinking water, aesthetically unacceptable drinking water will undermine the confidence of consumers. It will also lead to complaints and more importantly possibly lead to the use of water from sources that are less safe.
The study of hydrogeochemical processes in groundwater helps to understand and distinguish between the rock-water interactions and anthropogenic influences. The geochemical processes occuring within the groundwater and the reaction with aquifer minerals have a profound effect on water quality [3] . Groundwater chemically evolves by interaction with aquifer minerals or internal mixing of different groundwaters along subsurface flow-paths [3] [4] . Therefore spatial distribution of chemical species gives some idea about the direction of groundwater movement.
Because of the variety of variables observed as groundwater quality data, and uncertainty involved in trans- port and reaction mechanism into groundwater systems, it is necessary to implement a sophisticated knowledge extraction and diagnosis tool that can provide the analysis and visualisation of multidimensional groundwater quality data. Generally, multivariate analysis methods such as factor analysis and principal component analysis [3] [5] have been used for this purpose in hydrological and groundwater systems. Due to the rapid innovation of computer technology, the artificial neural network technique, particularly self-organising maps (SOM), which is a powerful tool for multivariate, nonlinear analysis and modeling, has recently attracted considerable attention in analysis and diagnosis of dynamic systems. One reaction for this may be the increasing demand for efficient multivariate and nonlinear techniques to be applied in intelligent systems for optimal monitoring and diagnosis of dynamic systems.
Oumé Department in Côte d’Ivoire has limited sources of safe drinking water with the major reliable source being groundwater. Nevertheless, the rapid development of this area leading to increase in the population estimated to 3.8% per year brought about a drastic increase in water requirements, which might be in the long term become problematic [6] .
The water need of the community of Oumé Department is satisfied by many boreholes and privates wells from the weathered and fractured aquifers. The quality of the water can be affected by natural processes and human activities such as indiscriminate refuse and waste disposal, the use of septic tanks, soak-away pits. Water treatment and integrity of distribution pipes can also affect the quality of drinking water obtained from groundwater [7] .
Although considerable information has been accumulated about the weathered and fractured aquifers in Oumé region from previous studies [6] [8] , no systematic studies have been done to evaluate the influence of natural processes and human activities on groundwater quality.
This work provides a better understanding of the hydrogeochemical quality of waters from the weathered and fractured aquifers in Oumé region.
2. Material and Methods
2.1. Study Area Description
The study area is located in the Centre western of Côte d’Ivoire in Oumé Department. It covers the area between latitude 6˚10' and 6˚40'N and longitudes 5˚10' and 5˚50'W. It is approximately 285 km2 in area (Figure 1).
The geological bedrock consists of the volcano-sedimentary and the granitoides, which are essentially consti- tuted by granites and granodiorites. On the one hand, the volcano-sedimentary includes flyschoides constituted
of flyschs. Schists, meta-sediments and undifferentiated rocks. On the other hand, the volcano-sedimentary is covered by metavulcanites which consist of amphibolites, andesite, spilites, conglomerates and grauwackes. Fracturing is more developed in the volcano-sedimentary complex than on the granitoides [9] [10] .
From the hydrogeological point of view, the most important aquifers are the fractured aquifers of crystalline and schist rocks. The transmissivity and specific yield ranged from 1.08 × 10−6 to 1.28 × 10−3 m2∙s−1 and from 0.01 to 2.11 m2∙h−1 respectively [6] .
2.2. Groundwater Samples
Groundwater was sampled from 91 locations (11 wells and 80 boreholes) during the long rainy season of 2009 (July). The sampling points were selected based on the geographic location of wells and boreholes and the use of the wells and boreholes as sources of drinking water. Figure 2 shows the location of the selected wells and boreholes.
Samples from wells were collected with weighted buckets (50 cm below the water table). For boreholes, samples were taken after pumping for 5 min. The tap and the bucket were cleaned before sampling and caution was taken to avoid splashing. Samples for analysis of cation contents were acidified for stabilization using sulphuric acid.
Samples were collected in 500 mL polyethylene bottles. Once collected, all samples were stored on ice and immediately transported to the laboratory. Chemical analyses were processed within 24 h after collection.
2.3. Physicochemical Analyses
The measured parameters (nitrate, hydrogenocarbonate, sulphates, chloride, calcium, magnesium, sodium, po-
![]()
Figure 2. Location of sampling points. Sampling points B16, B17, B42, B44, B51, B54, B68, 72; B8, B9; B27, B28; B29, B31, B33; B22, B23, B24, B25, B26, B32; B4, B5; B7, B19; B41, B78; B38, B56; B40, B65; B2, W2; B11, B39; B15, W10; B12, W6; B10 and W4 are located in 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15, respectively.
tassium and iron) were determined at the laboratory according to the methods presented in Table 1. Water temperature (T˚), electrical conductivity (EC), pH, dissolved oxygen (DO) and turbidity (Turb) were measured in situ using the HACH Model 44,600 Conductivity Meter (for T˚ and EC), the Multi 340i handheld (for pH and DO) and the HACH Model 2100P Turbidimeter (for Turb). Classification and interpretation of the groundwater hydrochemistry was carried out using the Piper Diagram.
2.4. Pattern Analysis of the Groundwater Qualities Due to the Mineralization
The data set collected was used to analyze the effect of the hydrogeochemical processes on groundwater quality. The data set collected has 15 variables or components. These 15 components in the data file were used to create the self-organizing map (SOM). The 9 × 6 size of the map is used and the total number of nodes (54) in the hexagonal grid is displayed. After a map has been created, the component planes are placed serially to analyze the dependencies between components. In each component plane, each hexagon represents one map node and its colour tells the value of the component in that node. Hexagons in each place on different components planes correspond to the same map node and show the values of the components in the weight vector of that node. Each component plane window represents the local average component value at each node in a certain colour.
3. Results and Discussion
3.1. Groundwater Quality
Physicochemical data of the collected water samples are summarized in Table 2. These data were also compared with World Health Organization (WHO) standards (Table 3).
The pH of the groundwater remained acid (4.27 - 7.10) for both the weathered and fractured aquifers. It also appeared that groundwater acidity was higher in the weathered aquifer than in the fractured aquifer. In agree- ment with [11] , this difference of acidity may be explained by the CO2 production in the topsoil under the action of the biological activities. Indeed, the study area abounds many primary forests in protected forest areas. The presence of these forests promotes the abundance of plant organic matter. Its mineralization releases CO2 which
![]()
Table 1. Analysis methods of chemical parameters.
![]()
Table 2. Statistics of the physicochemical parameters of groundwater samples from wells and boreholes in Oumé Department.
is dissolved in groundwater as follows:
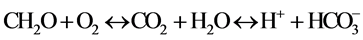
No health-based guideline value has been proposed for pH [16] . However these lower-pH groundwaters are more likely to be corrosive. Failure to minimize corrosion can result in the contamination of drinking-water and in adverse effects on its taste and appearance.
Water electrical conductivity (EC) of these two studied aquifers varied from 35 to 1071 µS/cm and showed that the prospected wells and boreholes were weakly to fairly mineralised. This may be related to the nature of silicate rocks within the groundwater from the two studied aquifers. It is established that the geochemical processes occurring within the groundwater and the reaction with aquifer minerals have a profond effect on water mineralisation.
It has been noted that groundwater EC values were higher in the weathered aquifer than in the fractured aquifer. This result differs from those obtained by [12] in Odienné area (north-west of Côte d’Ivoire), [13] in Man area (western of Côte d’Ivoire), [14] in Daloa area (Côte d’Ivoire) and [15] in Benin. These authors obtained the highest EC values in the fractured aquifer. This suggests that in the study area there is no connection between
![]()
Table 3. WHO drinking water standards (WHO, 2011).
the weathered aquifer and the fractured aquifer.
Compared with the acceptability of drinking-water guideline proposed by [16] , water of the two-studied groundwater presented low concentrations of major elements (Ca2+, Mg2+, Na+, Cl−, 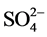 and K+) (Table 2). They were low hardness (from 9.6 to 209.7 mg∙L−1 of Ca2+ and Mg2+), low salt (from 1.2 to 94.3 mg∙L−1 of Na+), low chlorinated (from 1.2 to 89.8 mg∙L−1 of Cl−) and presented low concentrations of sulphate (from 0 to 74.9 mg∙L−1 of
and K+) (Table 2). They were low hardness (from 9.6 to 209.7 mg∙L−1 of Ca2+ and Mg2+), low salt (from 1.2 to 94.3 mg∙L−1 of Na+), low chlorinated (from 1.2 to 89.8 mg∙L−1 of Cl−) and presented low concentrations of sulphate (from 0 to 74.9 mg∙L−1 of ). According to [16] , the health-based guideline for nitrate in drinking-water is 50 mg∙L−1.
). According to [16] , the health-based guideline for nitrate in drinking-water is 50 mg∙L−1. 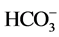 concentrations of all the samples were below the permissible limit except at borehole F27 (69.1 mg∙L−1). The implication of this is that the water had very little contamination with landfill leachate, domestic sewage and other sources of pollution.
concentrations of all the samples were below the permissible limit except at borehole F27 (69.1 mg∙L−1). The implication of this is that the water had very little contamination with landfill leachate, domestic sewage and other sources of pollution.
The levels of iron detected ranged from 0.01 - 1.16 mg∙L−l. Seventy-eight sampling sites presented iron concentrations lower than 0.3 mg∙L−1 (Table 2). According to [16] , there is usually no noticeable taste at iron concentrations below 0.3 mg/L, although turbidity may develop as it has been noted in some sampling sites such as boreholes B28 (8.27), B30 (3.58), B31 (11.3) and B34 (5.23). The sampling sites that have concentrations of iron above 0.3 mg∙L−1 are: B6 (0.36), B21 (1.1), B22 (0.5), B23 (0.48), B24 (0.48), B25 (0.71), B26 (1.16), B27 (1.12) and W7 (0.57). At levels exceeding 0.3 mg/L, iron in waters of these boreholes and wells stains laundry and cause taste.
The low values obtained for these chemical characteristics indicated that the water may be potable, safe and fit for drinking.
3.2. Hydrogeochemical Processes
Figure 3 shows a dominance of the major ions  and Ca2+ in the weathered and fractured aquifers while other ions such as K+, Mg2+ and
and Ca2+ in the weathered and fractured aquifers while other ions such as K+, Mg2+ and 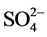 are comparatively less represented. Concentrations of major cations and major anions are classified as :
are comparatively less represented. Concentrations of major cations and major anions are classified as :
 and
and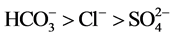 . Thus, the Piper’s diagram indicates well waters and most of borehole waters belong to the calcic bicarbonated (Ca-HCO3) type (Figure 4).
. Thus, the Piper’s diagram indicates well waters and most of borehole waters belong to the calcic bicarbonated (Ca-HCO3) type (Figure 4).
The hydrochemical facies obtained in this study is consistent with those of [17] . These authors investigated quality of groundwater in aquifers of crystalline and crystallophyllian rocks in Man areas in Côte d’Ivoire. According to these authors, the Ca-HCO3 type is a major feature of groundwater from aquifers of crystalline and crystallophyllian rocks.
The  ion is mainly due to acid hydrolysis of rocks [14] . This hydrolysis is accompanied by the disso-
ion is mainly due to acid hydrolysis of rocks [14] . This hydrolysis is accompanied by the disso-
![]()
Figure 3. Mean concentration of chemical parameters of groundwater sam- pled in Oumé Department.
![]()
Figure 4. Piper diagram showing the groundwater chemical types of the study area.
lution of cations in particular Ca2+ and Mg2+ which are the most mobile in the weathering of rocks [17] [18] .
A few samples showed different trends as calcic chlorided (Ca-Cl) and sodium-calcium-chloride (Na-Ca-Cl) type of hydrochemical facies (Figure 4). This indicates some localized changes in groundwater quality. In agreement with [3] and [19] , this is possible only in case of anthropogenic input in groundwater system. These findings are in agreement with the component planes and the SOM for the study area displayed in Figure 5. The nodes that represent the high values (mean logarithmical values) are in red and those representing the low values are coloured blue. Indeed, three clusters are extracted from the SOM.
The statistical descriptions of each cluster are shown in Table 4. It is noted that group A is characterized by the high values in Ca2+, Mg2+, K+, Na+ and![]() . By comparing component plane, component planes of these cations have similar distributions over the map with the hydrogenocarbonate plane. It means that cations compo- nents in this group are highly positively correlated with hydrogenocarbonate (Table 5). Negative relationship is also observed between acid-pH and hydrogenocarbonate. These correlations demonstrate that the alteration of acid rocks followed by the dissolution of cations is the predominant mechanism of mineralization in this group. Group B corresponds to low mineralization with high dissolved oxygen and low values of Ca2+, Mg2+, K+,
. By comparing component plane, component planes of these cations have similar distributions over the map with the hydrogenocarbonate plane. It means that cations compo- nents in this group are highly positively correlated with hydrogenocarbonate (Table 5). Negative relationship is also observed between acid-pH and hydrogenocarbonate. These correlations demonstrate that the alteration of acid rocks followed by the dissolution of cations is the predominant mechanism of mineralization in this group. Group B corresponds to low mineralization with high dissolved oxygen and low values of Ca2+, Mg2+, K+, ![]() , Fe, Cl−,
, Fe, Cl−, ![]() and
and![]() . The comparison of hydrogenocarbonate abundance and potassium, calcium and magnesium concentrations shows that there is not generally a positive relationship between these cations and hydrogenocarbonate (Table 5). This effect may be explained by water in this group in contact with hardly alterable acid rocks, hence the low mineralization of waters observed. These correlations also may mean that
. The comparison of hydrogenocarbonate abundance and potassium, calcium and magnesium concentrations shows that there is not generally a positive relationship between these cations and hydrogenocarbonate (Table 5). This effect may be explained by water in this group in contact with hardly alterable acid rocks, hence the low mineralization of waters observed. These correlations also may mean that
![]()
Table 4. Statistical description of each cluster found in the study area.
Cond: conductivity; T˚: temperature; Turb: turbidity.
![]()
Table 5. Spearman correlation coefficients (r) between hydrogenocarbonate and physicochemical parameters in groundwater of the area study.
*p < 0.05 for 0.222 ≤ │r│ < 0.549; **p < 0.01 for │r│ ≥ 0.549.
water mass whose renewal is easy. Waters of group C are characterized by high conductivity, high nitrate. sulphate and phosphate concentrations. This shows that the phenomena of anthropogenic pollution are predominant in the mecanism mineralization waters of this group.
Effects of water-rock interactions were investigated by calculating calcite (CSI) and dolomite (DSI) saturation indices. As shown in Figure 6, all well waters in the weathered aquifer and most of borehole waters from fractured aquifer are under-saturated to dolomite and to calcite. On the one hand this effect may explain the lower residence time of water in the aquifers. Hence, the gathering together of almost all water wells and boreholes may indicate a lack of connection between both weathered and fractured aquifers. Consequently their supply to water spread out at different ways. On the other hand the distribution of waters in Figure 5 may reveal the discrimination of wells and boreholes in contact with different geological formations on the basis of rocks basicity and acidity. Previous studies [17] [18] have reported that the mineralization and Ca2+, Mg2+ and pH values were higher in groundwater in contact with basic rocks than in the acidic rocks. In this study, it also appears that the values of Ca2+, Mg2+, pH and the mineralization of waters low when calcite and dolomite saturation indices are low. Therefore, the water distribution could be based on the nature of the rocks ranging from a basic pole with
![]()
Figure 5. Component planes and SOM visualizations for the study area.
![]()
Figure 6. Graph of calcite and dolomite saturation indices.
![]() to an acid pole (
to an acid pole (![]() ). These assumptions show the complexity of the hydraulic operating environments crystalline basement.
). These assumptions show the complexity of the hydraulic operating environments crystalline basement.
4. Conclusions
In Oumé Department, groundwaters in the weathered and fractured aquifers are used for irrigation and domestic purposes. The present study shows a significant impact of hydrogeochemical processes on the quality of these groundwaters. Hydrochemical evolution of the waters is largely controlled by alteration process of acid and basic rocks followed by the dissolution of ions, and locally by anthropogenic effects. Careful examination of the relationships between hydrogenocarbonate and other hydrochemical parameters was useful to understand not only the hydrochemical evolution but also the origins of major ions.
This study also indicates that the water supply met many of the criteria for good quality water. We suggest that a systematic, hydrogeochemical and environmental isotopic study is essential to understand the groundwater system and to prepare an appropriate measure for water quality protection
NOTES
*Corresponding author.