Preparation Characterization and Thermal Behaviour of Carbopol-TiO2 Nanocomposites ()
1. Introduction
Hybrid organic-inorganic materials have been widely studied in the last decade [1] -[3] and polymer matrix-ce- ramic filler composites receive increased attention due to their interesting optical, electrical and electronic prop- erties [4] - [8] . Furthermore it is well known that hybrid materials have high thermal and chemical stabilities, long life and appreciable proprieties in their different applications. These proprieties made them desirable for indus- trial application in food, pharmaceutical and electronic industries. So we can consider that the incorporation of inorganic nanoparticles into polymer matrix can considerably affect the proprieties of the matrix. These hybrid materials are mixture of organic and inorganic groups at a molecular level this is why their proprieties became dependent on both organic and inorganic parts. Consequently the obtained composite might provide improved thermal and mechanical ability. Not without standing, the proprieties of these composites depend on the incor- poration manner of the nanoparticles, their size, shape, concentration and the nature of their interaction with the polymer matrix. Carbopol is considered as a common and successful polymer matrix for the fabrication of composite materials due to its good adhesion with reinforcing elements [9] . Titanium dioxide TiO2 is an inex- pensive, non toxic compound with remarkable optical and electrical proprieties [10] - [12] . This is why it has been the subject of the interest of many researchers, who spent a great deal of effort to realize useful materials for industrial application. However the potential application of the ceramic filler composites at elevated temperatures, required more studies and further improvement in the materials preparation.
On the basis of this consideration we report in the present work a convenient manner to prepare carbopol- TiO2 nanocomposites using a heating process. A number of analyses were selected as XRD, SEM and FTIR spectroscopy to obtain information on the structure and the morphology of these novel materials. Additionally the influence of the TiO2 nanoparticles on the thermal proprieties of carbopol matrix was investigated using thermo-gravimetric analysis, TGA and differential scanning calorimetry DSC.
2. Experiment
Carbopol-940 is the name of a commercial polymer composed of long chains of polyacrylic acid cross-linked with allyl sucrose, with a carboxylic acid content not less than 56% and not more than 68% and molecular weight higher than 4 × 106 g∙mol−1. The powdered form of these polymers, obtained from Fluka, was used as polymer matrix, and TiO2 powder was purchased from Aldrich. The DMF and acetone were purchased from Fluka and all reagents were used as received without further purifications.
2.1. Synthesis of TiO2-Carbopol Nanocomposites
Carbopol-TiO2, composites, were synthesized by dissolving separately 1.2 g of carbopol and 0.6 g of titanium dioxide in DMF solvent. The suspensions were stirred at room temperature under constant magnetic stirring during 3 h. Then the system was slowly heated, with a constant rate of 1˚C/min, up to 30˚C, 45˚C, 60˚C, 80˚C, 100˚C and 120˚C. A control sample was taken at each temperature. The reaction was allowed to proceed at the last temperature for 3 h. The colloidal solutions were left to cool down at room temperature. After cooling, the samples were dispersed with acetone. Then composites were isolated by centrifugation, washed with the same solvent and dried in a vacuum for 2 weeks, at 30˚C, to ensure total elimination of residual solvent then stored in desiccator to prevent moisture absorption. The obtained materials were named T30, T45, T60, T80, T100 and T120, according to the temperature at which they were extracted 30˚C, 45˚C, 60˚C, 80˚C, 100˚C and 120˚C respectively.
2.2. Characterization of Carbopol-TiO2 Nanocomposites Samples
A JSM-6300 scanning electron microscope (SEM) (JEOL Ltd., Japan) was used to investigate the surface mor- phology of the CP-TiO2 nanocomposites samples after (Au) coating. The anatase structure of TiO2 was studied using X-ray diffraction (XRD). The (XRD) patterns of the samples were recorded on a Rigaku (D/MAX-2200 Ultima/PC) diffractometer with Cu Ka Reflectance (ATR) Spectrum-one Perkin-Elmer spectrophotometer. The spectra were recorded at room temperature and the samples were analyzed from 5˚ to 80˚ (2θ˚). CP-TiO2 nano- composites were characterized by Fourier transform infrared (FTIR) spectroscopy, on a Universal Attenuated Total temperature from 650 to 4000 cm−1, with a resolution of 2 cm−1. A background spectrum without any sample was subtracted from all spectra. The glass transition temperature (Tg) of the nanocomposites were meas- ured with a TA Instrument 2010, DSC2 differential scanning calorimetry (DSC), using a sample amount of 10 - 15 mg, with a heating rate 20˚C/min, under nitrogen flow. The initial onset of the change of slope in the DSC trace during the second heating scan was identified with the sample Tg. Thermo-gravimetric (TG) and Differen- tial thermo-gravimetric (DTG) measurements were performed using Dupont Thermal analyser 2000. About 10 mg of sample was heated in a platinum crucible from ambient to 800˚C. At a heating rate of 10˚C/min.
3. Results and Discussion
3.1. Surface Morphology by SEM Analysis
Scanning electron spectroscopy, SEM, provided very useful information about the particle size and location of ceramic fellers on the surface of the material. Figure 1 shows SEM images of CP-TiO2 nanocomposites T45, T100 and T120. The titanium dioxide with a globular structure and a high homogeneity and surface area are clearly visible as a small spots which adhered to the CP matrix, probably due to the hydrophilic propriety of TiO2 and the strong affinity between the later and the carboxylic groups of the carbopol.
3.2. X-Ray Diffraction Study of the Carbopol-TiO2 Nanocomposites
Figure 2 gives the X-ray diffraction (XDR) patterns of the doped ceramic fellers nanocomposites, T30, T40, T60, T80, T100 and T120. The peaks exhibited at 2θ values 27.13˚, 35.83˚, 38.33˚, 44.67˚ and 54.34˚ are in good agreement with those corresponding to the tetragonal anatase structure of TiO2 (25.36˚, 37.85˚, 38.64˚,
![]()
![]()
![]()
Figure 1. SEM images of carbopol-TiO2 nanocomposites T45, T100 and T120.
![]()
Figure 2. X-ray patterns of carbopol-TiO2 nanocomposites.
48.15˚ and 53.97˚) as reported by ASTM data and are assigned to reflections through to the positions (101), (103), (004), (200) and (105) respectively. This result constitutes a proof of the presence of nano-sized TiO2 par- ticles in the analyzed materials, additionally to the agglomerated particles seen by SEM analysis.
3.3. Optical Characterization
An alternative technique used to confirm the existence of TiO2 nanoparticles into the polymeric matrix was the Uv-visible absorption spectroscopy. The absorption spectra of the pure carbopol and the CP-TiO2 nanocompo- sites are presented in Figure 3. While no peak appeared in the carbopol absorption spectrum in the region 245 to 360 nm (not shown), the absorption spectra of the nanocomposites showed a large band centered at 315 nm, re- lated to the large distribution size of TiO2 clusters evidenced by SEM analysis. Additively we can see another band that appeared around 274 nm corresponding to the nano-sized TiO2 nanoparticles, which are clearly evi- denced by XRD patterns.
3.4. ATR-FTIR Characterization
The FTIR spectra of pure components CP and CP-TiO2 nanocomposites in the hydroxyl region from 3800 cm -1 to 2200 cm -1 are shown in Figure 4(a). It is well known that CP corresponds to cross-linked polyacrylic acid polymer and similarly to the PAA polymer it shows typical intermolecular hydrogen bonds at 3170 cm -1 (bonded hydroxyl) and at 3526 cm -1 (free hydroxyl) [9] -[13] .
In the FTIR spectra of CP-TiO2 nanocomposite, the band at 3170 cm -1 decreased in intensity while the band at 3526 cm−1 increased and shifted to lower vibration 3418 cm -1. The decrease of the band at 3170 cm -1, corre- sponding to the carboxylic intramolecular hydrogen bonding, is caused by the creation of novel interaction be- tween the hydroxyl groups of CP and TiO2 nanoparticles. The shift of the band corresponding to the free hy- droxyl groups from 3526 cm -1 to 3418 cm -1, indicates that the OH…TiO2, bonding is formed. Furthermore, the important shift on this band (Dν = 108 cm-1), indicates the existence of strong interactions between the CP hy- droxyl groups and the titanium dioxide nanoparticles.
We can also see in this region two peaks related to the methylene and methyne groups that appeared at 2980 cm -1 and 2929 cm -1 in the CP. These two peaks shifted to lower wavenumbers 2929 cm -1 and 2855 cm-1 respec- tively in the CP-TiO2 nanocomposite. This result is probably related to the modification of the electronic envi- ronment of the methylene and methyne groups caused by the interaction between the CP carboxylic hydroxyl groups and the titanium atoms. See Scheme 1.
Furthermore, the satellite band enhanced by Fermi resonance, that appeared at 2619 cm−1, in the pure CP spectrum shifts significantly to the low frequency side at 2500 cm−1 in the CP-TiO2 nanocomposite. This dis- placement reflects a redeployment of a part of the self-associated acid groups to hetero-associated ones.
In the carbonyl region from 1800 to 1350 cm -1, the CP shows an intense and broad band centred at 1724 cm -1
![]()
Figure 3. Uv-visible absorption of carbopol and carbopol-TiO2 nanocomposites obtained in spectroscopic DMF solvent.
![]()
Figure 4. ATR-FTIR spectra of carbopol and carbopol-TiO2 nanocomposites (a) in the hydroxyl; (b) in the carbonyl; and (c) in the Ti-O bonds, representative regions.
with a shoulder at 1702 cm -1 see Figure 4(b). These bands correspond to the acid-acid intermolecular and in- tramolecular associations respectively. In the CP-TiO2 nanocomposites samples, the carbonyl band centred at 1720 cm -1, shifts to lower wavenumbers at 1702 cm -1, indicating the break of acid-acid intermolecular hydrogen bonding and the appearance of a new band centred at 1640 cm -1 attributed to the complex formation between carboxylic groups and TiO2 nanoparticles in the CP-TiO2 nanocomposites.
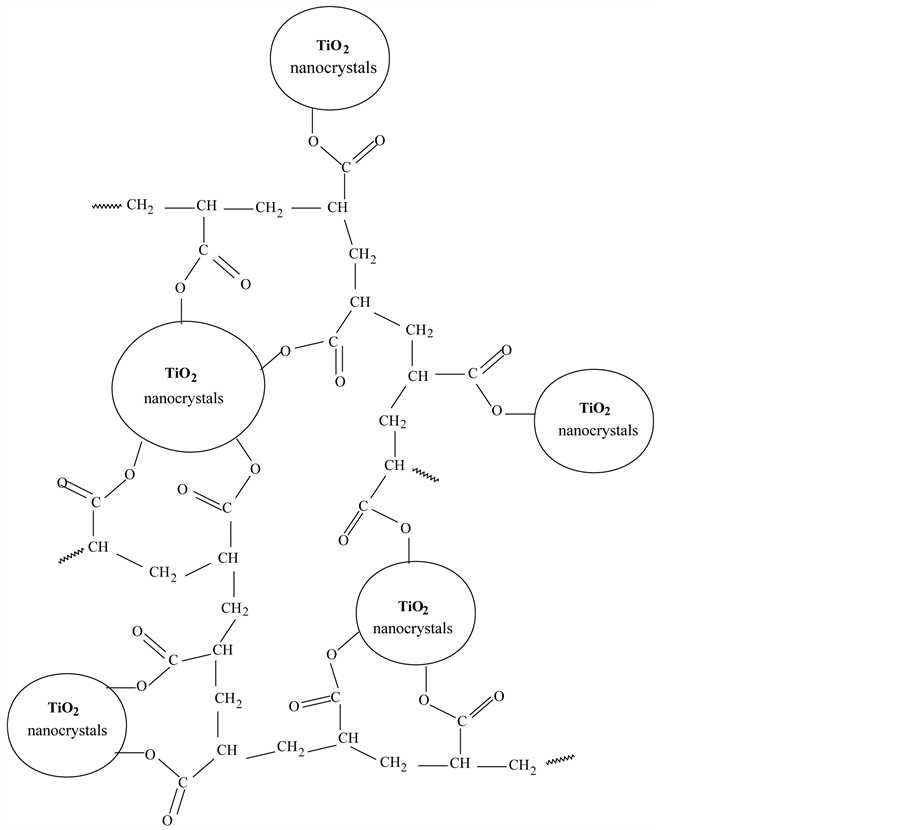
Scheme 1. Schematic structure of the ester like linkage between carbopol and TiO2 nanocrystals as predicted by FTIR and ATG analyses.
Furthermore, Figure 4(b), shows the appearance of symmetric and asymmetric vibration signals of carboxy- late anions, situated at 1416 and 1542 cm−1, assigned to the protons transfer from the carboxylic groups, of CP to the titanium dioxide, predicting the formation of ionic interactions between carboxylate and titanium ions.
Figure 4(c) presents the FTIR spectra of CP-TiO2 composites, from 1500 to 700 cm−1. The most interesting bond from IR spectra of TiO2―nanocomposites are the absorption peak at 1004 cm−1 and 1070 cm−1 which may correspond to Ti-OH stretching motions and Ti-O-C bending, respectively [14] . The Ti-O-C may result from the interaction between the Ti-O-C network and the aliphatic chain of carbopol groups. Therefore we can expect stable bonding between the organic and the inorganic components.
Figure 4(c) also shows that the characteristic absorption peak of anatase 1630 cm−1, shifts to higher wa- venumber, about 1642 cm−1, in the carbopol-TiO2 composites. These results reveal that the composites are not the simple mixtures of polymer and TiO2 nanocrystals. These should be strong interactions between the CP car- boxylic groups and nanoparticles, may be new chemical bonds are formed on the interface between CP-TiO2 nanocomposites and TiO2 nanocrystals. See Scheme 1.
3.5. Differential Scanning Calorimetry Analysis
The DSC thermograms of pure carbopol and CP-TiO2 nanocomposites display a single glass transition tempera- ture (Tg), as it can be seen in Figure 5. All glass transition temperatures value of the nanocomposites are higher then Tg = 127˚C of the initial carbopol polymer. Moreover there value increase slightly from T30 to T120 nanocomposites from 156.61˚C to 183.69˚C. These high Tg value are related to the strong interactions between TiO2 nanoparticles and the polymer matrix which acted as physical cross-links, involving the reduced segmental mobility, hence leading to the increasing the glass transition temperature. Then we can conclude that the pres- ence of TiO2 nanoparticles within the carbopol matrix increase the glass transition temperature of the initial CP polymer.
3.6. Thermo-Gravimetric Analysis
The thermo-gravimetric (TGA) and derivative thermo-gravimetric (DTG) curves of pure CP and CP-TiO2 nano- composites samples obtained at a rate of 10˚C∙min−1 are presented in Figure 6. As it can be seen, the degradation process of CP, fellows three successive degradation stages at (25˚C - 100˚C, 100˚C - 400˚C and 400˚C - 590˚C). The first stage of degradation is mainly related to the water loss corresponding to 7.56% of weight loss that occurred at 100˚C, which might be accompanied by anhydride formation through the polymer chains. The second stage that occurred at higher temperature is attributed to the release of CO2 and CO gas, by decarboxyla- tion process and finally the last stage was related to the main chains scissions [15] [16] . One must note that complete degradation of CP occurred at 577˚C leading to 0% of residual material at 574˚C. By the other size, CP-TiO2 composites thermograms also showed three degradation stages at (27˚C - 275˚C, 275˚C - 420˚C and 420˚C - 600˚C) which appeared slightly at higher temperatures.
The results of the thermal degradation of the CP-TiO2 nanocomposites T30 to T100, showed less than 0.4% of weight loss at 100˚C, since the pure carbopol sample gives 7.56% weight loss at the same temperature. Fur- thermore these nanocomposites exhibit less than 3.8% weight loss till 200˚C. According to this important find- ing we can conclude that the introduction of ceramic fellers in carbopol matrix leads to the improvement of its thermal proprieties and moreover blocked the water loss that might occur at 100˚C. We can therefore relate this phenomenon to the modification of the structure of the CP matrix by introducing TiO2 nanoparticles as predicted by FTIR study.
Thermal proprieties of the CP were really improved in the CP-TiO2 nanocomposites since the value of the de- gradation temperature (Td) of the main degradation stage increases from 538˚C in the pure CP to 573˚C in T100.
By the other seize, we must also note the diminution of the Td and Tg temperatures in the nanocomposite T120. This result may be related to some degradation that probably occurs during the synthesis process at 120˚C in the carbopol matrix.
![]()
Figure 5. DSC diagrams of carbopol and carbopol-TiO2 nanocomposites.
![]()
Figure 6. (a) Thermo-gravimetric and (b) differential thermo-gravimetric curves of carbopol and carbopol-TiO2 nanocomposites.
Previous work of Dzunuzovic et al. on the thermo-gravimetric degradation of TiO2 nanoparticules encapsu- lated in PMMA matrix showed that, during the degradation process, the reaction between the oxygen and tita- nium dioxide may happen in two ways [17] . The first way of the reaction may occur on the material surface. The second way of the reaction occurs in the bulk of the material. The surface reaction usually occurs at relatively low temperature (300˚C and 500˚C) while the bulk reaction takes place at higher temperature (700˚C - 900˚C). According to that we related the degradation stages between 275˚C - 420˚C and 600˚C - 800˚C in the CP-TiO2 nanocomposites curves to the reaction between the oxygen of titanium dioxide that might happen on the surface and on the bulk of the materials respectively.
Another point which might to be highlighted is the high level of the residual materials obtained in the CP- nanocomposites. An average of 35.65% of char yield was obtained in the synthesized materials at 600˚C. Since 0% of residual material is obtained for pure CP. The incomplete degradation of the nanocomposites is probably a result of thermal cross-linking between TiO2 and polymer bonds, induced by heating the samples during ther- mo-gravimetric analysis. Nevertheless the char yield can be a decisive factor to estimate the limited oxygen in-
![]()
Table 1. Thermal and thermo-gravimetric parameters of carbopol and carbopol-TiO2 nanocomposites.
a,bTemperature at which 5% or 10% weight loss was recorded by TGA analysis at a heating rate of 10˚C/min; c,dMain stage degradation temperature obtained by ATG and glass transition temperature obtained by DSC; eWeight percentage of material left after TGA analysis at maximum temperature 800˚C in N2; fLimiting oxygen index.
dex (LOI) of polymers according to Van Krevelen-Hoftyzer’s equation [18] [19] .
 (1)
(1)
where CR is the char yield.
The synthesized nanocomposites had an average value of LOI equal to 32.3. On the basis of the LOI values, such macromolecules can be classified as self-extinguishing polymers. The thermo-gravimetric parameters of all the samples investigated in this work are summarized in Table 1.
4. Conclusion
In this present study we successfully prepared the new CP-TiO2 nanocomposites. Results showed that TiO2 nanoparticles maintained their tetragonal anatase-type crystalline structure in the carbopol matrix as evidences by XDR patterns. Furthermore Uv-visible analysis confirmed their nano-sized characters. The FTIR study allowed us to identify the strong associated bonds that exist between the TiO2 nanoparticles and the CP carboxylic groups. Differential scanning calorimetry analysis showed that the presence of TiO2 nanoparticles within the carbopol matrix increase the glass transition temperature of the polymer. The thermal stability of the nanocomposites was studied on the basis of the weight loss of the materials at 100˚C and the residue at 800˚C. The results showed that however 10% of degradation occurs at 100˚C for the pure carbopol, the carbopol-TiO2 nanocomposites remained stable till 200˚C with an average weight loss, less than 3.8% at this same temperature. Results also showed that the nanocomposites have dependence on the temperature at which they were extracted since the value of their glass transition temperature increased gradually from 127.08˚C in the pure carbopol to 183.69˚C in T120 nanocomposite. These materials with good thermal stability could be considered as new processable high-performance polymeric materials.
NOTES
*Corresponding authors.