Endosulfan Causes Neoplastic Changes in the Liver Cells of Mice ()
1. Introduction
Food problems have haunted mankind since time immemorial. With few technology breakthrough to increase yields, the food needs of growing population were historically met by expanding the cultivated area. It is also true that the green revolution has succeeded in transforming the Indian economy from a situation of severe food shortage into one where the country has not only become self-reliant in food production but has also been able to generate a sizable surplus for export. India is a predominantly agrarian country with about 60% - 80% rural population. Pesticides are routinely used for advanced farming to improve crop yield and for control of different pests. The term pesticide covers a wide range of compounds including insecticides, fungicides, herbicides, rodenticides, molluscicides, nematocides, plant growth regulators and others. Among these, organochlorine (OC) insecticides, used successfully in controlling a number of diseases, such as malaria and typhus, were banned or restricted after the 1960’s in most of the technologically advanced countries. Ideally a pesticide must be lethal to the pests, but not to non-target species, including man. Unfortunately, this is not so the controversy of use and abuse of pesticides has surfaced. The rampant use of these chemicals, under the adage, “if little is good, a lot more will be better” has played havoc with human and other life forms [1] .
Among various organochlorines, Endosulfan is widely used in India. Endosulfan is classified in India as an “Extremely Hazardous” pesticide [2] , “Moderately Hazardous chemical” by (WHO-class II), highly toxic substance [3] [4] and moderately hazardous pesticide after taking LD50 value [5] . Recently, Government of Kerala, India, banned this pesticide (Endosulfan) after its hazardous effect on the reproductive health of Kassaragod district cashew nut workers. Most of the cases related to male and female infertility and abnormal child birth were found. It was also confirmed by many authors [6] -[11] .
Since, Endosulfan exposure had caused lots of health hazards among the cashew nut workers in Kerala, India it thence became a rationale to re-evaluate its carcinogenic effect in animal models. Endosulfan leads to liver carcinogenesis is the novel work ever added in this area. It is similar to the carcinogenic effects observed by many clinicians in cashew nut workers of Kassargod district of Kerala [12] . The present study thus, illustrates the carcinogenic activity of Endosulfan on the liver cells of Swiss albino mice.
2. Materials & Methods
2.1. Animals
For the study female Swiss albino mice were procured from the animal house of Mahavir Cancer Institute & Research Centre, Patna, Bihar, India (CPCSEA Regd. no. 1129/bc/07/CPCSEA, dated 13/02/2008) and their ethical approval for this study was obtained by the Institutional Animal Ethics Committee (IAEC). The age group of female mice for the experiments was 12 weeks old. The average body weight of experimental mice was 30 ± 2 gm. All the female mice were acclimatized for one week prior to the experimentation. The animals had free access to water and feed pellets (prepared mixed formulated feed by the laboratory itself) [13] .
2.2. Test Pesticide
The commonly used pesticide-Endosulfan (Excel India Pvt. Ltd. Mumbai with EC 35%) was obtained from the local pesticide market of Patna.
2.3. Experimental Design
The animals were grouped into two groups, Group I, control n = 6, received distilled water as drinking water while Group II, Endosulfan treated group (n = 24) received endosulfan 3 mg/kg b.w daily by gavage method for 4 weeks after estimation of LD50 value which was found to be 7 mg/kg body weight. Then, the animals were left without treatment for 6 months to observe any adverse effect. After 6 months, the animals were sacrificed and their liver tissues were fixed in respective fixatives.
2.4. Histopathology & Transmission Electron Microscopy
All mice were sacrificed after the scheduled period. A midsaggital incision was made and liver tissue from all the mice were removed and fixed in 10% neutral formalin for light microscopy study. For Transmission Electron microscopy it was fixed in 2.5% gluteraldehyde in 0.1M sodium phosphate buffer (pH 7.2) with in 24 h of removal. After rinsing with phosphate buffer, tissues were postfixed with 2% osmium tetraoxide in sodium phosphate buffer. Dehydration was accomplished by gradual ethanol series and tissues were embedded in epoxy resin. Semithin sections were stained with toluidine blue and examined with a light microscope (Olympus, LXi, Tokyo, Japan). Ultrathin sections (800 nm) were stained with uranyl acetate and lead citrate. Sections were then viewed and photographed with Morgagini - 268 D TEM (SEI Co.) at SAIF-EM facility Unit (Sophisticated Analytical Instruments Facility) at All India Institute of Medical Sciences (AIIMS), New Delhi, India. For the light microscopic study, the Haemotoxylin-Eosin stained slides were prepared and the sections were viewed under light microscope.
3. Results
3.1. Morbidity and Mortality
The mice after Endosulfan exposure (3 mg/Kg b.w./day) for 4 weeks have shown signs of toxicity such as nausea, nose bleeding, lack of body co-ordination, general body weakness and loss of body weight. But, in the mice which were left for 6 months, there was further significant decrease in the bodyweight denotes the degree of toxicity.
3.2. Histpathological Findings
Hepatocytes, sinusoids and sinusoidal cells structures in Group I (control group) were normal as normal architecture of hepatocytes well arranged near central vein were observed (Figure 1). In Group II (Endosulfan treated group) hepatocytes with haemorrhaged central vein and bile duct were observed. Vacuolations in sinusoidal spaces with numerous Kupffer’s cells were also observed. The most important finding is the neoplastic growth as observed in the section (Figure 2) denotes the malignancy in the hepatocytes.
3.3. Transmission Electron Microscopic Findings
In the transmission electron microscopic examination, degeneration in hepatocytes in Endosulfan treated group was evident as pseudopodia like formation of nuclear membranes of two nuclei were observed, denotes high degree of degeneration (Figure 3) in comparison to the control hepatocyte section (Figure 4). Rupture in the nuclear membranes with dilations in the nuclear pore complex and their movement into the cytoplasmic region is clearly observed (Figure 5) with formation of neoplastic bodies (Figure 6). The highly significant result showed the migration of cytoplasmic material into the nucleus (Figure 7) i.e. nuclear inclusion denotes the neoplastic condition as the nuclear inclusion shows mitochondria with rough endoplasmic reticulum (Figure 8). Free lying polyribosomes in cytoplasm also denote the neoplastic condition in the hepatocytes.

Figure 1. Light microphotograph (Haemotoxylin-Eosin (H&E) stained) section of control liver showing normal architecture of central vein (CV) with hepatocytes (H) well arranged in sinusoids (S). ×800.

Figure 2. Light microphotograph (Haemotoxylin-Eosin (H&E) stained) section of Endosulfan treated liver showing degeneration in the hepatocytes (H) with haemorrhage in central vein (CV) and bile duct (BD). Vacuolations in sinusoidal spaces with numerous Kupffer’s cells and neoplastic growth (NG) in the section are also clearly visible. ×500.
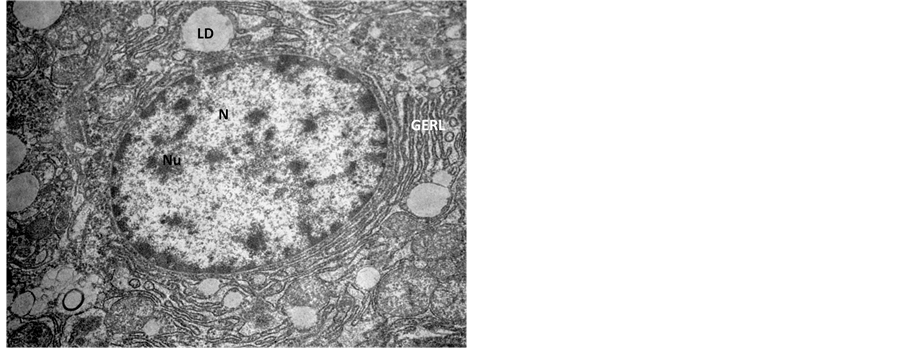
Figure 3. Transmission Electron micrograph section of control liver showing normal architecture of nucleus (N) with nucleolus (Nu), the Golgi pathway (GERL) and lipid droplets (LD). ×14,000.

Figure 4. Transmission Electron micrograph section of Endosulfan treated liver showing hepatocytes with pseudopodia like formation of nuclear membrane of the two nuclei (N). The dilated nuclear pore complex (NPC) denotes high degree of degeneration. ×56,000.

Figure 5. Transmission Electron micrograph section of Endosulfan treated liver showing hepatocyte with degeneration in rough endoplasmic reticulum (RER) and mitochondria (M). Ruptures in nuclear membrane, while migration of nuclear pore complexes (NPC) in to the cytoplasmic region, denotes high degree of degeneration. ×28,000.

Figure 6. Transmission Electron micrograph section of Endosulfan treated liver showing hepatocyte in degenerative condition with neoplastic body (NB) 28,000.

Figure 7. Transmission Electron micrograph section of Endosulfan treated liver showing peculiar condition of nuclear inclusion (NI) containing RER and mitochondria (M). Rupture in the nuclear membrane and degenerated cytoplasm is observed. ×14,000.

Figure 8. Transmission Electron micrograph section of Endosulfan treated liver showing magnified view of Figure 7, showing nuclear inclusion (NI). The nuclear inclusion contains mitochondria (M), RER with ruptured nuclear membrane (NM), free lying polyribosomes (PR) in the cytoplasm. ×56,000.
4. Discussion
Endosulfan is an organochlorine pesticide causing major damage to the liver injury. Peroxidation of membrane lipids and formation of free radicals are the major cause responsible for the toxic effects of Endosulfan. Under normal conditions, the free radical levels in body are low and the healthy organisms can ameliorate or neutralize their effects by free radical scavangers such as antioxidants. Lipid peroxidation, particularly those containing polyunsaturated fatty acids can significantly change the properties of biological membranes. In the present study, Endosulfan has caused high degree of degeneration at cellular as well as sub cellular levels. The haemorrhages in central vein, bile duct and neoplastic growths in hepatocytes denotes neoplastic condition while degeneration in rough endoplasmic reticulum (RER) leads to cleavage of ribosomes from the RER making in free lying polyribosomes in the cytoplasm. The membranes have lost their integrity leading to cyto-nucleic pressure causing migration of cytoplasmic materials into the nucleus i.e. nuclear inclusion. This is the indicator of neoplasia as this type of pattern is generally observed in neoplastic cells [14] .
In the other studies on Endosulfan, it was observed that it causes degeneration in hepatic cells of rats [15] -[19] . Impact of endosulfan on fish at histopathological and biochemical and haematological levels have also been well studied [20] -[25] . In the present histopathological study also, it was observed with many structural damages, neoplastic growth in the hepatocytes, degeneration in cytoplasmic organelles and nuclei of the hepatocytes. Molecular mechanisms underlying the carcinogenic effects of endosulfan in human liver showed phenotypical effects of endosulfan on HepG2 liver cells. Endosulfan disrupted the anoikis process and cells exposed to endosulfan were initially sensitized to anoikis and thereafter recovered their resistance to this process. Altogether, the results indicate that endosulfan profoundly alters the phenotype of liver cells by inducing cell detachment and partial EMT as well as disrupting the anoikis process. All these events account, at least in part, for the carcinogenic potential of endosulfan in liver [26] . In a parallel study, it has been reported that endosulfan exposure causes ovarian malignancy in Swiss albino mice [27] . Endosulfan had not only led to cause liver cancer among cashew nut workers but also of throat, blood and uterus [12] . Endosulfan is probably responsible of causing gall bladder cancer in humans in Gangetic belt of eastern and western India has been well documented [28] . Thus, from the entire study, it is revealed that endosulfan exposure to experimental animals causes hepatic injury at great extent leading to neoplastic changes in the hepatic cells.
Acknowledgements
The authors are thankful to SAIF-EM facility Unit (Sophisticated Analytical Instruments Facility) at All India Institute of Medical Sciences (AIIMS), New Delhi, India and Mahavir Cancer Institute & Research Centre, Patna, Bihar, India for providing infrastructural facilities.