Leaf Morphological and Stomatal Variations in Paper Birch Populations along Environmental Gradients in Canada ()
1. Introduction
Plants typically express phenotypic differences in response to environmental changes [1] -[3] . Under different environmental conditions, plants allocate biomass in several organs in order to capture optimum light, water, nutrient and carbon dioxide, and as a strategy to maximize growth rate [4] . Phenotypic plasticity also occurred to produce a range of leaf characteristics as a response to environmental effects [5] . These differences in plants particularly at leaf levels are expressed as morphological and anatomical variation.
Leaf morphological and anatomical variation in plants growing in contrasting habitat (i.e. climatic gradient) has long been studied [1] [3] . Leaf morphological studies show that narrow and thick leaves provide structural reinforcement to withstand wilting in hot, sunny and dry environments [6] -[8] . Additionally, it is suggested that small leaves track air temperature closely, whereas large leaves suffer from overheating when water is limited [2] . A study on the adaptive significances of leaf hairs showed an increase in leaf reflectance, boundary layer thickness and prevention in stomatal obstruction by water or particulate matters [9] [10] . Consequently, increased leaf hairs in hot and arid habitats have significant influence in reducing solar radiation, leaf temperature and transpirational losses [9] -[11] . Hence, the most commonly observed leaf morphological changes under water deficiency are reduced leaf area [12] and specific leaf area [12] [13] and increased leaf hairiness [10] .
Alternatively, even in abundant water availability the cost for replenishing transpired water is high because of investment in the root and vascular network to transport water [14] [15] . Thus, the most noticed leaf anatomical adaptation to high water transportation cost is stomatal evolution [16] -[20] . Stomata in plants regulate gas exchange under environmental constraints. Leaf stomata optimize between photosynthetic gain and transpirational loss to adjust in precipitation and temperature fluctuations [21] . It is suggested that smaller stomatal area and guard cells increase carbon dioxide diffusion per unit area of stomata and reduce water loss compared to larger stomatal area and guard cells [6] . As an adaptation mechanism to water stress, others show that stomatal density increased in Pseudoroegneriaspicata [22] whereas stomatal area decreased in Pistaciaatlantica [23] .
Most of the studies on leaf morphological and stomatal variation in response to environmental variables have either included comparative studies among multiple species [7] [16] [24] or species inhabiting different locations along environmental gradient [6] [7] [25] -[27] . Results of these studies showed marked genetic variation, adaptive significance and phenotypic plasticity in leaf morphology and stomata or both. However, leaf morphological and stomatal variations for multiple species inhabiting different environment do not necessarily explain the variation at intraspecific level. Therefore, it is important to determine whether leaf morphology and stomata differ in wide-ranging pioneer species like paper birch (Betula papyrifera Marsh.) grown in a uniform environment. To our knowledge, no studies have focused on leaf morphological and stomatal variations of the birch populations grown in a uniform environment.
Paper birch adapts to a wide range of climatic and soil moisture regimes in North America, and the species is increasingly significant in commercial forestry [28] . The birch populations may have developed leaf morphological and stomatal variations that have allowed them to adapt to a wide climatic gradient. In this study, we addressed whether leaf morphology and stomata differ among paper birch populations that originate from different environments but grown in the same environment, and whether differences in leaf morphology and stomata are related to the environmental variables of a population’s origin. We hypothesized that: (1) leaf morphological and stomatal characteristics vary among the birch populations grown in the uniform environment; (2) leaf characteristics are related to the environmental variables of the populations’ origin; (3) the population that originate from warmer regions with less precipitation has smaller leaf area or high leaf hair density; (4) the population that originate from a region with higher precipitation and aridity index has lower stomata density or higher stomatal area; and (5) significant positive relationships exist among stomatal density, stomatal area and leaf hair density.
2. Materials and Methods
2.1. Sample and Leaf Morphological and Stomatal Data Collection
Seeds of sixteen paper birch populations were collected from Ontario, British Columbia, New Brunswick, Newfoundland, Nova Scotia, Prince Edward Island and Quebec. The populations’ origins ranged from 10 - 840 metres elevation, 1639 - 279 mm mean annual precipitation and 1.36˚C - 8.88˚C mean annual temperature across Canada (Table 1). The birch seedlings were grown for 12 weeks in Lakehead University’s greenhouse. In August 2008, thirty uniform seedlings in height and root-collar diameter from each population were selected and transplanted in the common garden in Thunder Bay, Ontario (located at 183.3 meters above sea-level, and 48˚22ꞌN and 89˚19ꞌW). The layout of the populations were following completely randomize design in the garden.

Table 1. Latitude (Lat.), longitude (Long.), elevation (Elev.) in meters, mean annual precipitation (MAP) in millimeter, mean annual temperature (MAT) in degrees Celsius, mean annual aridity index (MAI), precipitation during growing season (GSP) in millimeter, temperature during growing season (GST) in degrees Celsius, aridity index during growing season (GSA) of sixteen paper birch populations collected (seeds) from across Canada and grown at the common garden in Thunder Bay—Ontario.
We randomly harvested leaves from middle of the crown of the birch populations in the common garden in August 2010. Eight healthy, well-developed leaves from each population were collected on randomly chosen individuals for leaf morphological and stomatal analysis following the methods of Warren et al. [2] and Hovenden and Schoor [29] respectively. Leaf morphological characteristics such as leaf area and aspect ratio (horizontal width/vertical length of leaf) were measured using WinFolia software (Regent Instrument Inc. Quebec, Canada). Hair densities on abaxial and adaxial leaf surfaces were counted on three parts (0.20 cm2) of each leaf surface using Academic stere zoom microscope at 30× magnification and average values were used for further analysis [2] [30] [31] . Subsequently, the leaves were dried at 70˚C for 42 h to calculate specific leaf area.
Stomata were absent on the adaxial leaf surface; therefore stomatal replicas were assessed and analyzed for abaxial surface only. We obtained stomatal replicas from middle section of leaves by using clear nail varnish [26] . Leaf veins were avoided as far as possible while collecting stomatal impressions. We used electronic microscope and motic images plus 2.0® software (Motic Instruments Inc., Richmond, Canada) to obtain photos of stomata. We measured stomatal density (stomata/1 mm2 i.e., 106 µm2), length, width, pore area and guard cell width per leaf for further analysis [16] [32] . The equations used for calculating stomatal characteristics are listed in Table 2 [16] [33] -[35] .
2.2. Climate Variables
Mean annual and growing season temperature and precipitation data for population’s origin were normalized climate data from 1971 to 2001 (Weather-Environment Canada). We calculated the mean annual and growing season annual aridity index using De Martonne’s [36] [37] and Sijors’s [38] equations respectively (Table 2).

Table 2. Equations used for leaf morphological and anatomical characteristic; and climate variables of paper birch populations here, SLA: specific leaf area (cm−2·gm−1), LDM: leaf dry mass (gm), LS: leaf area (cm2), SA: stomatal area (µm2), SL: stomatal length (µm), SW: stomatal width (µm), SD: stomatal density, ED: epidermal cell density, PA: stomatal pore surface area (µm2), PL: pore length (µm), PW: pore width (µm), SI: stomatal intensity, SHC: stomatal shape coefficient, MAI: mean annual aridity index, MAP-mean annual precipitation (millimeters), MAT: mean annual temperature (˚C), GSA: mean aridity index during growing season, GSP: mean precipitation during growing season (millimeters), GST: mean temperature during growing season (˚C) and Nv: length of growing season (days).
2.3. Data Analyses
We analyzed the variations in both leaf morphological and stomata characteristics using nonparametric test because of the relatively small sample size for each population [2] . Variations in leaf morphological and stomatal characteristics were analyzed using the Kruskal-Wallis test. We used Mann-Whitney U test to analyze the morphological and stomatal difference between populations. Correlation between measured leaf characteristics and climate of paper birch population’s origin were analyzed using Spearman’s correlation. We analyzed the relationship between stomatal and morphological characteristics using Spearman’s correlation. All statistical analysis was conducted using SPSS-18 (SPSS, Chicago, IL, USA) and R-2.12.1 (R-Development Core team 2011).
3. Results
3.1. Variations in Leaf Morphological and Stomatal Characteristics
Leaf area, specific leaf area, aspect ratio and leaf hair density showed significant variation among the paper birch populations (P ≤ 0.05) (Table 3). Populations from Porcupine Lake, Wayerton, Pettawa and Adam Lake had significantly larger leaf area in comparison to Newfoundland, which had the smallest leaf area (Table 3). Populations from Wayerton and Milvale had significantly smaller specific leaf area compared to populations from Adam Lake, Porcupine Lake and Skeena River (Table 4). Skimikin had lower leaf abaxial and adaxial hair densities that significantly differed from Adam Lake, Porcupine Lake and Skeena (N = 16, P ≤ 0.01) (Table 3).
Apart from stomatal index and shape coefficient, analyses of stomatal length, width, and size, pore length, pore width, pore area, stomatal density and guard cell width showed significant differences (N = 16, P ≤ 0.01) among paper birch populations (Table 3, Figure 1). Populations from Newfoundland and Skimikin differed from the majority of populations in stomatal area, pore area and stomatal density. The population from Skimikin had significantly smaller mean stomatal area and higher stomatal density (N = 16, P ≤ 0.05) than those from Adam Lake and Porcupine (Table 3, Figure 1). The population from Adam Lake had the lowest stomatal density, but the largest stomatal area, pore area and guard cell width (Table 3, Figure 1).
3.2. Correlation between Leaf Characteristics and Climate of Population’s Origin
We found significant correlation between measured leaf characteristics and environmental variables of the paper birch population’s origin (Table 4). Leaf area and aspect ratio were positively related to longitude (r = 0.29 and 0.33 respectively, P ≤ 0.001), elevation (r = 0.26 and 0.51 respectively, P ≤ 0.001) and growing season temperature (r = 0.33 and 0.26 respectively, P ≤ 0.001); however, they were negatively related to annual and growing season precipitation (r = −0.33 and −0.42 respectively, P ≤ 0.001) and aridity index (r = −0.36 and −0.44 respectively, P ≤ 0.001; Table 5). Specific leaf area increased from north to south (latitude) (r = 0.43, P ≤ 0.001) and east to west (longitude) (r = 0.33, P ≤ 0.001), however, it decreased with increasing growing season temperature (r = −0.29, P ≤ 0.001), precipitation (r = −0.31, P ≤ 0.001) and aridity index (r = −0.26, P ≤ 0.001; Table 4).

Table 3. Results of the Kruskal-Wallis nonparametric tests of leaf morphological and stomatal characteristics (chrs) of 16 paper birch populations collected (seeds) from across Canada and grown at the common garden in Thunder Bay—Ontario.

Table 4. Spearman’s correlation between leaf characteristics (Chrs.) and climatic of paper birch populations’ origin. The values are correlation coefficient and N = 16.
Here *, ** is significant at P < 0.05, P < 0.001 respectively; ns: not significant.

Figure 1. Intraspecific variations in stomatal area and pore area among paper birch.

Table 5. Spearman’s correlation coefficient (with p value in parenthesis) within of leaf morphological and stomatal characteristics (Chrs.) of 16 (N) paper birch populations collected (seeds) from across Canada and grown at the common garden in Thunder Bay—Ontario.
Here *, ** is significant at p < 0.05, p < 0.001 respectively; ns: not significant.
We found low hair density on leaf abaxial surface along increasing mean annual and growing season temperature gradients (r = −0.26, P ≤ 0.001and r = −0.21, P ≤ 0.01 respectively). Similarly, we found less hair density on adaxial surface with increasing mean annual precipitation (r = −0.23, P ≤ 0.001) and aridity index (r = −0.30, P ≤ 0.001) (Table 4).
Longitude, and growing season precipitation and aridity index of paper birch population’s origin range from 57.57 - 128.34, 29.55 - 100.08 mm and 3.08 - 13.5, respectively (Table 1). All stomatal characteristic measured, except for stomatal shape coefficient, were significantly correlated with longitude, growing season precipitation and aridity (P ≤ 0.05) (Table 4). Both stomatal area and guard cell width increased with longitude (r = 0.35 and 0.35, respectively, P ≤ 0.001) and a decrease in growing season precipitation (r = −0.33 and −0.32, P ≤ 0.001 respectively) and aridity index (r = −0.37 and −0.37 respectively, P ≤ 0.001) (Table 4). On the contrary, stomatal density decreased with an increase in longitude from east to west (r = −0.36, P ≤ 0.001), and the density was related to a decrease in mean annual and growing season precipitation (r = 0.24, p = 0.03 and r = 0.38 respectively, P ≤ 0.001) and aridity index (r = 0.39 and 0.38 respectively, P ≤ 0.001) (Table 4).
Along temperature gradient, we found that stomatal area and guard cell width were positively correlated to mean annual temperature (r = 0.29 and 0.30 respectively, P = 0.01) (Table 4). Similarly, stomatal area increased with increasing growing season temperature (r = 0.22, P = 0.05) whereas, the stomatal shape coefficient decreased with an increase in the temperature (r = −0.23, P = 0.04). We found larger pore (area) in warmer temperature (r = 0.30, P ≤ 0.01) associated with decreased precipitation (r = −0.36, P ≤ 0.001) and aridity index (r = −0.38, P ≤ 0.001) during the growing season (Table 4). However, we found no significant correlation between stomatal index and the climate of the populations’ origin. Furthermore, none of the stomatal characteristics were significantly related to latitude and elevation of the origin.
3.3. Correlation between Leaf Morphological and Stomatal Characteristics
Within a leaf morphological characteristic, we found significantly higher hair density on adaxial surface in larger leaf area (r = 0.21, P = 0.01) and aspect ratio (r = 0.26, P = 0.001) (Table 5). Within stomatal characteristics, increase in stomatal density significantly decreased stomatal area (r = −0.72, P = 0.001) (Figure 2), pore area (r = −0.68, P = 0.001) and guard cell width (r = −0.56, P ≤ 0.001) (Table 5) Comparing leaf morphological and stomatal characteristics, we found that in stomatal density was more in smaller leaf area (r = −0.56, P = 0.03) and specific leaf area (r = −0.51, P = 0.05) with less hair densities on abaxial (r = −0.65, P = 0.01) and adaxial (r = −0.85, P ≤ 0.001) surfaces (Figure 3). Adaxial hair density was more in leaves with larger stomatal area (r = 0.64, P = 0.01), pore area (r = 0.55, P = 0.03) and guard cell width (r = 0.63, P = 0.01). However these stomatal characteristics were insignificantly related to other leaf morphological characteristics (Table 5).
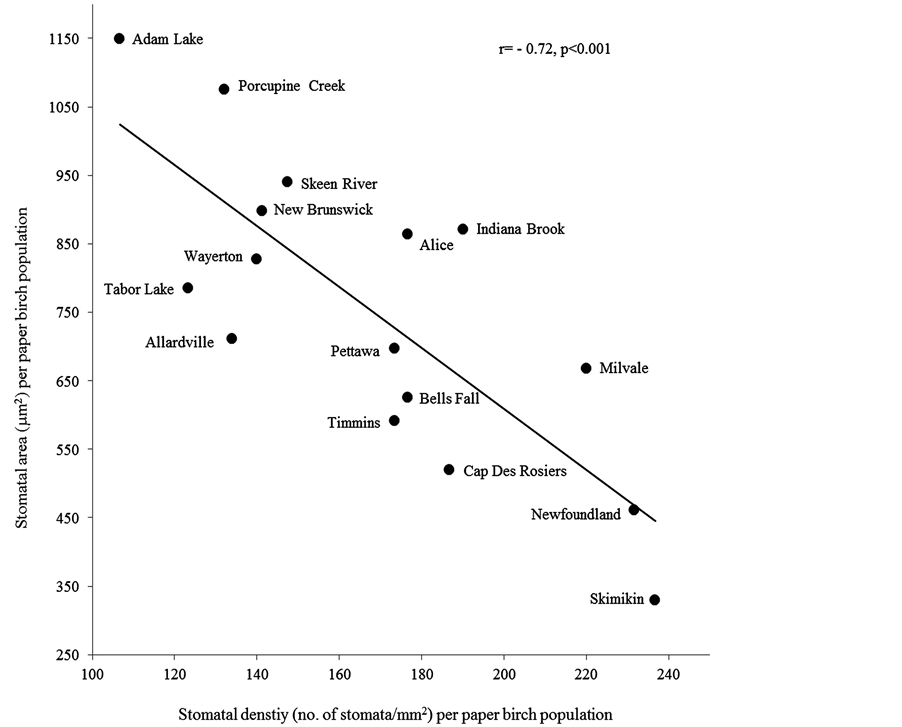
Figure 2. The correlation between stomatal density and size (area) for sixteen paper birch populations.
4. Discussion
The results of this study demonstrate significant variations in leaf morphological and stomatal characteristics of paper birch populations grown under uniform conditions in a common garden. The patterns in the variations are consistent with the results of other studies on Quercus petraea and Parkia biglobosa [1] [30] . The population variations observed in this study suggested that morphology and stomatal characteristics of the birch populations were maintained under a uniform environment, except for stomatal index and stomatal shape coefficient. This therefore supports the hypothesis that the birch populations significantly vary in leaf morphological and stomatal characteristics. These variations may be related to genotypic differences. It is possible therefore that environmental difference at population’s origin identified among genotypes in this study and elsewhere [2] [39] had contributed to leaf variation in the paper birch populations.
We tested if genotypic differences in the leaf characteristics were related to the environment of the paper birch population’s origin. Supporting our hypothesis, the results showed that the leaf characteristics that varied in the paper birch populations were significantly related to longitude and climate of the birch population’s origin. Species show wide mechanism of adaptation to water deficiency such as reduction in leaf area, specific leaf area and aspect ratio [40] and/or increase in leaf hairiness [41] . Consistent with previous studies and supporting our third hypothesis, our results showed that paper birch leaves had higher adaxial hair density with decreasing annual precipitation and aridity index. Conversely, larger and wider leaf area was noticed in elevational, longitudinal and temperature (during growing season) gradients with decreasing precipitation and aridity index. Although our result did not support the hypothesis that smaller leaf originates in warmer region, the strong positive correlation between hair density on leaf adaxial surface and leaf area may explain reducing evapo-transpiration
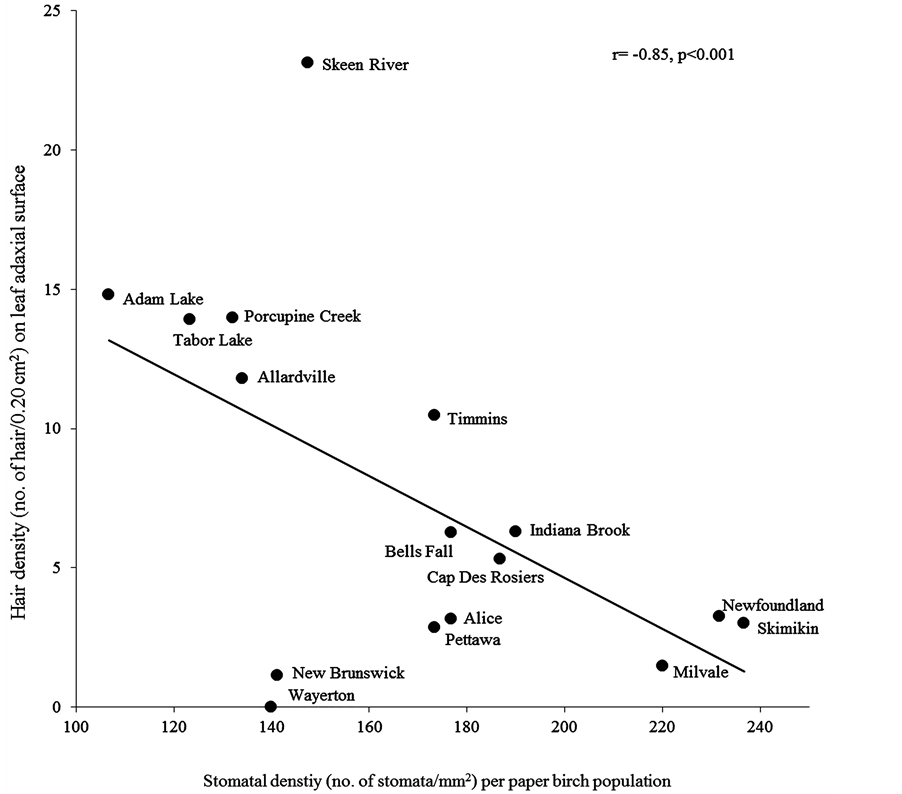
Figure 3. The correlation between mean stomatal density and leaf hair density on adaxial surface for sixteen paper birch populations.
from larger leaves in water deficiency. Similar to our result, most studies on intraspecific variation show an inconsistent relationship between leaf traits and climatic variables in comparison to interspecific variation. For instance, a study on Cistus salviifolius found bigger leaves in a drier area [42] whereas the opposite trend was found in Cistus ladanifer [43] . Similarly, an inconsistent relationship was found between leaf morphology of red ironbark and rainfall of different population origins [2] . The study on Cistus salviifolius demonstrated that leaf traits of plants grown in different conditions such as greenhouse generally differ from those in natural populations [42] , which might be the case in our common garden study on paper birch.
In our study, longitude and aridity index (both mean annual and growing season) were major environmental variables that were significantly related to stomatal characteristics of paper birch. Stomatal area and density characterize species’ resistance to drought [23] [44] . Small stomatal area with higher density was noticed in Populus trichocarpa from xeric environments [45] and stomatal density increased in Lolium perenne under elevated temperature [46] . In contrast to these studies, we found that paper birch had larger stomatal area and pore area with lower density in relation to decreasing precipitation and aridity index during the growing season. Even though our results did not support our hypothesis, it is consistent with a study on paper birch populations from water deficit sites that had larger and fewer stomata per unit area [47] .
The tradeoff between stomatal area and density; that is, either larger stomatal area with low density or smaller stomatal area with high density, revealed by the strong correlations in our study, is consistent with other studies [18] [48] . Although stomatal area reduced with precipitation and aridity gradients in this study, stomatal area per unit leaf area remained unchanged due to an increase in stomatal density. Similar to our result, temperate species from drier habitats also had smaller stomatal area and higher stomatal densities that were associated with higher stomatal conductance [7] . Alternatively, larger stomatal area and lower stomatal density in deciduous tree species was associated with a slow increase in stomatal conductance under unfavorable conditions, such as warmer temperature [24] . Thus, we conclude that the tradeoff between stomatal area and density and their correlation to the climate of origin of the paper birch populations might be a strategy by the species to balance stomatal conductance in drier habitats. Furthermore, stomatal density and index revealed a negative relationship with increasing longitude, whereas stomatal area was positively related to longitude. Hence, populations that originated on the west coast had fewer stomatal densities with larger stomatal area in comparison to populations from the east coast. In the present study, we found no significant relationship between stomatal density and elevation, which was consistent with other studies [19] [49] . Results from previous studies on either increase or decrease of stomatal density to increased elevation were contradictory [50] . Moreover, in our study, stomatal area was positively correlated with the mean annual temperature of the origin which was consistent with previous studies [51] . Others suggest that small stomata can open and close more rapidly and would increase rapid stomatal conductance to maximize CO2 diffusion into the leaf, during favorable conditions [18] .
Under environmental stress such as water deficiency, plants develop traits that either diminishes the loss of water or traits that reduce the need for water by increasing water use efficiency [52] . Small leaves with hairs could reduce transpiration by lowering leaf temperature or by changing boundary layer conditions [53] [54] . Furthermore, if small leaves had fewer stomata water use efficiency for a species will change. Although we did not subject the populations to any stress, increased precipitation and aridity index during the growing season at the origin positively increased stomatal density and decreased stomatal area, leaf area, specific leaf area and aspect ratio. Supporting our last hypothesis, our result showed significant correlations among stomatal density, size and leaf characteristics. Correlations between leaf morphological and stomatal characteristics revealed that populations with larger leaf area, specific leaf area and higher hair density had low stomatal density. Furthermore, populations with higher hair density on the adaxial surface had larger stomatal area, pore area and guard cell width. All these features provide a structural basis in reducing water loss through leaves and water use efficiency. However, we did not measure water use efficiency in this study. Nevertheless, leaf morphological and stomatal studies are valuable for identifying ecologically important traits that can then be further analyzed in other experiments [55] [56] .
5. Conclusion
In conclusion, our results raise the possibility that intraspecific variation in paper birch might evolve due to genotypic variation and environmentally induced variation in leaf morphological and stomatal characteristics. Contrary to our expectations, several leaf characteristics were less related to environmental gradient of the birch’s origin. Yet, we should consider the fact that the common garden was located at the climatic condition (mean annual precipitation 711 mm, temperature 2.25˚C and aridity index 74.73) that was different than the environment the populations would normally be exposed to. Thus, phenotypic plasticity of the birch possibly has imposed leaf characteristics contrary to our expectations to acclimate in the common garden environment [57] . Further studies involving the use of greenhouse experiment in plants grown under different environmental conditions are necessary to better understand how morphology and stomata vary in paper birch populations across Canada and their possible phenotypic plasticity to a changing climate.
Acknowledgements
The research was funded by NSERC discovery grant to JW. We appreciate Mrs. J. Lee, Profs M. Leitch and L. Hutchion for logistic support during greenhouse and laboratory experiments. We are grateful to Prof. A. Reif, Mr. S. F. Bartels and anonymous reviewers for their feedbacks.