Bi-Enzymatic Conductometric Biosensor for Detection of Heavy Metal Ions and Pesticides in Water Samples Based on Enzymatic Inhibition in Arthrospira platensis ()
1. Introduction
In the open environment, humans and animals placed in the food chain and in particular the marine food chain are exposed to heavy metal poisoning due to the ability of these pollutants to concentrate via the food chain. Present in traces, the toxicity of heavy metals becomes a serious threat to human health when they bioaccumulate in micro-organisms, thus inducing biomagnification. Pesticides have been widely used for decades in agriculture, medicine and industries as they are highly efficient as insecticides and herbicides; they are highly neurotoxic and carcinogenic [1] [2] .
These harmful effects highlight the need for instituting a rapid systematic and continuous monitoring of pollutants. Electrochemical platform detection [3] -[8] and biosensors are such devices that can be implemented. A lot of works has been done with electrochemical, piezoelectric or optical transducers for the measurement of pesticides through enzyme inhibition [9] -[17] . These biosensors use either cholinesterase (ChE) in conjunction with choline oxidase (ChO) or only AChE [18] [19] . Biosensors for the detection of heavy metals are based on enzyme inhibition of phosphatase activity (PA) and urease [20] -[23] . The majority of these biosensors are based on immobilized pure enzymes. These enzymes cannot be immobilized on the same sensor easily because of their different optimal operational conditions. Arkhypova et al. [21] have proposed a multibiosensor based on enzyme inhibition for pollutant detection. However, they have faced stability problems when immobilizing several enzymes on a multi-detection array. This is why an emerging device for toxic substances monitoring is the enzyme electrode, but it still has limitations related to simplicity of use, stability, portability and cost. Micro-organisms can be a good bioreceptor for multi-detection. Recently, the development of whole-cell biosensors has attracted increasing interest. Microalgae were used in various studies to develop whole-cell biosensors for the control of toxic pollutants in aquatic environments [5] -[7] [24] -[28] .
Enzymatic activities, esterase activity (EA) [29] and phosphatase activity [30] are present in the same micro-organism which is Arthropira platensis [29] [30] . Arthropira platensis, called Spirulina, is a Gram negative bacterium. It is also considered as a blue-green microalga. These enzyme activities can be inhibited by two families of pollutants in order to obtain a bi-enzymatic biosensor: phosphatase activity is inhibited by heavy metals and esterase activity is inhibited by pesticides. In this study, the kinetic proprieties of phosphate and esterase activities were directly determined using conductometric transduction.
Many works have been carried out with these conductometric biosensors for the detection of a variety of molecules [31] [32] . This type of biosensor presents many advantages over other types of transducers: it can be produced through inexpensive thin film standard technology, no reference electrode is needed, the transducers are not light sensitive and differential mode measurements allow the cancellation of a lot of interference [22] . Spirulina cells were immobilized on the working electrode of gold interdigitated transducers (IDTs) and on the reference electrode, only BSA was immobilized. Gold nanoparticles were integrated in the immobilization process. Their roles in the amplification of the measured conductance signal have been already demonstrated in [33] .
2. Experimental
2.1. Chemical and Biological Reagents
Disodium p-nitrophenylphosphate (pNPP) and acetylthiocholine chloride (AChCl) purchased from Sigma-Aldrich were used as the substrates for measuring APA and AChE activities respectively. They were dissolved in a buffer solution to prevent a change of the total conductivity of the measuring medium when adding the substrate. Sodium hydrogenophosphate and sodium dihydrogenophosphate were purchased from Sigma-Aldrich. Phosphate buffer was prepared by dilution in Milli-Q water to obtain a concentration of 5 mM (Na2HPO4 and NaH2PO4) and the pH was fixed at 5.2.
To inhibit APA, the stock solutions of cadmium (Cd2+) and mercury (Hg2+) were prepared from pure standard solutions purchased from Sigma-Aldrich. And to inhibit AChE, the stock solutions of parathion-methyl, paraoxon-methyl and triazine, were prepared from products purchased from Sigma-Aldrich. Stock solutions were stored at 4˚C and fresh dilutions were prepared, in 5 mM phosphate buffer, 15 min before each series of measurements.
Monodispersed gold nanoparticles were purchased from Sigma-Aldrich, their mean particle size being 20 nm.
Bovine serum albumin (BSA), glutaraldehyde (GA) (grade II, 25% aqueous solution) and the polyelectrolyte used in this study, Poly(allylamine Hydrochloride) (PAH) were purchased from Sigma-Aldrich.
Arthrospira platensis (Compere 1968/3786 strain) called Spirulina, was cultivated under sterile conditions in Zarrouk liquid medium containing: (g/L) NaNO3, 2.50; K2HPO4, 0.50; NaHCO3, 10.00; NaCl, 1.00; MgSO4·7H2O, 0.2; CaCl2·2H2O, 0.02; FeSO4·7H2O, 0.01. All salts were of analytical grade and were purchased from Acros Organics. The medium was adjusted to pH 9.0 using NaOH solution. Cultivation was conducted in 5 L Erlenmeyer flasks. Cultures were maintained at 26˚C ± 1˚C under air bubbling and continuously exposed to UV lamps (100 μmol·photon/m2·s). After that, the biomass was recovered by filtration, washed with physiological liquid (9 g·L−1 NaCl in UHQ water).to remove the nutrient salts, and then lyophilized. The resulting powder was protected from moisture by storage in a closed vessel at 4˚C. Spirulina was dispersed in 5 mM phosphate buffer and was then placed under ultrasound and filtered (0.8 µm) before analysis. As Spirulina is made of transparent cells stacked end-to-end and entrapped with a sheath forming a spiral filament, the sheath was broken through sonication and filtration in order to separate the individual cells. This last step was crucial for our studies based on the enzymatic activities of Spirulina.
2.2. Scanning Electronic Microscopy
The SEM images were captured using a Quanta TM 250 microscope (FEI) in degraded pressure mode (i.e. injection of a certain water pressure in the analysis chamber, in order to observe the cells under moist conditions). Cells needed to be pretreated before imaging. They were fixed with covalent links using glutaraldehyde solution (3%, v/v) purchased from Sigma-Aldrich, specially purified for use as an electron microscopy fixative. Spirulina-based biosensors were immersed in this solution for 45 min. Then, a series of dehydration steps in successive absolute ethanol solutions was applied for 10 min using increased concentrations (20%, 40%, 60%, 80%, and 100% ethanol).
2.3. Sensor Design
The conductometric transducers (figure 1(a)) were fabricated at the V.Ye. Lashkaryov Institute of Semiconductor Physics (Kyiv, Ukraine). Each of them consisted of two identical pairs of interdigitated thin film electrodes (IDT electrodes) (150 nm thick), fabricated by gold vapor deposition onto a non-conducting pyroceramic substrate (5 × 30 mm). The technique of sensor manufacturing has been reported previously [33] -[35] . A 50 nm thick intermediate Cr layer was used to improve the adhesion of Au to the substrate. Both the digit width and interdigital distance were 10 μm, and their length was ~1.5 mm. As a result, the sensitive area of each pair of electrodes was ~2.9 mm2 (figure 1(a)).
2.4. Conductometric Device
A biosensor analyzer consisting of a sensor block and an electronic measuring block (a portable four-channel biosensor analyzer) was developed in collaboration with the Institute of Electrodynamics of the National Academy of Sciences of Ukraine and it was reported by Dzyadevych et al. [35] . The sensor block consisted of a stand with a fixed block of holders; each holder was connected to the fingers of an appropriate conductometric biosensor. An electronic measuring block consisted of the following modules: a secondary transducer and a basic measurement-control module. A personal computer with specific software support was an integral part of the measuring block. Using a generator, a sinusoidal excitation signal with a frequency of 30 kHz and amplitude of 10 mV was applied to the two exciting electrodes. These conditions avoided faradic processes, double-layer charging, and polarization of the microelectrodes. The signals measured were processed using the lock-in amplifier method, i.e. they were filtered using a low bandwidth centered on the excitation frequency and then transmitted to the synchronous detection which provided the difference of conductivity between the working electrode and the reference electrode. In fact, this system transformed the local variations in conductivity to a change of the active conductivity which can be recovered as information at the output of the system, such as variations in the output signal versus time.
Measurements were performed at room temperature in a glass vessel of 4 mL volume. The biosensor was immersed gently into 5 mM phosphate buffer. A magnetic stirrer ensured solution homogeneity throughout the experiments. After signal stabilization, different quantities of the substrate were added in the measurement vessel. A rinsing step was performed between two successive measurements.
2.5. Electrochemical Impedance Spectroscopy (EIS) Measurements
The measurements were performed with an electrochemical impedance analyzer “Voltalab PGZ 402” (Hach-
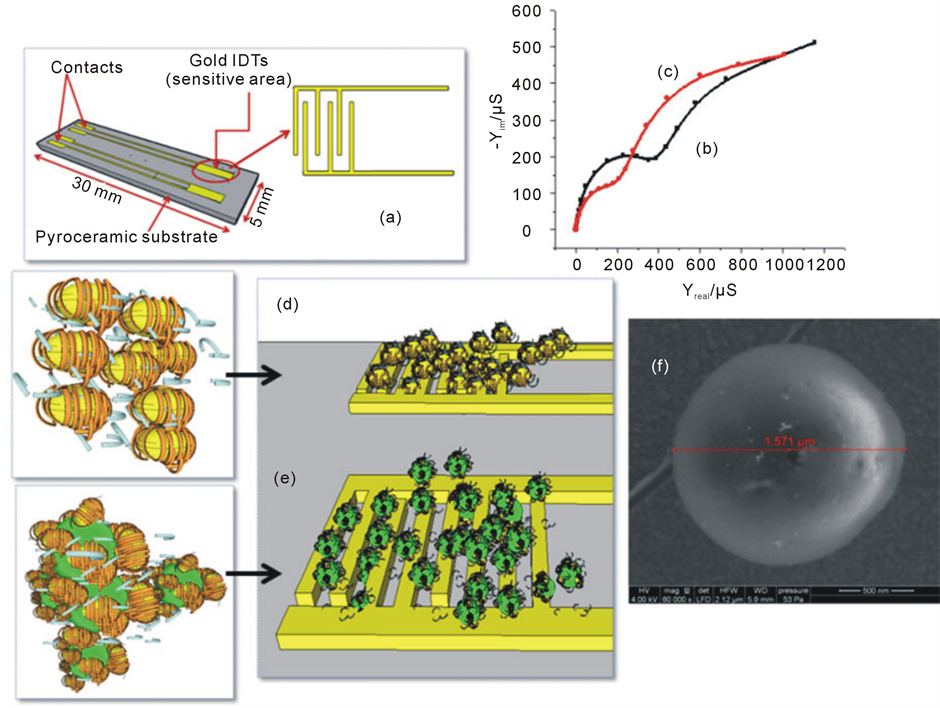
Figure 1. (a) Scheme of microelectrodes with gold IDTs [6] ; (b) Complex admittance spectra of bare electrode and (c) Modified electrode, where: frequency range = 100 mHz - 100 kHz, amplitude potential = 10 mV, with measurements made in phosphate buffer (5 mM at pH 5.2). (d) BSA (20%) mixed with PAH-coated gold nanoparticles, immobilized on the reference electrode, through cross-linking under glutaraldehyde vapours; (e) Spirulina cells mixed with PAH-coated gold nanoparticles and BSA (20%), immobilized on the working electrode, through cross-linking under glutaraldehyde vapours; (f) SEM image of Spirulina cell.
Lange, France) in a two electrode configuration. Data acquisition and processing via “Voltamaster” software was provided by the same company. All EIS measurements were taken at a frequency range of 100 mHz to 100 kHz at room temperature. The same glass vessel (4 mL) was filled with 5 mM phosphate buffer under vigorous magnetic stirring.
2.6. Preparation of PAH-Coated Gold Nanoparticles
A suspension of gold nanoparticles (1% w/w) was firstly centrifuged at 20˚C using a SIGMA 2 - 6 K Centrifuge from Fisher Bioblock Scientific at 9000 rpm for 20 min. The supernatant phase was then eliminated and the gold nanoparticles were immersed in 100 mL of aqueous solutions of 5 mg. mL−1 of PAH (positive charge), under mechanical stirring for 15 min. After the adsorption process, PAH-coated gold nanoparticles were removed by similar centrifuging and redispersion in 20 mM of phosphate buffer at pH 7.3.
2.7. Biosensor Functionalization
IDT electrodes were cleaned by sonication in acetone for 10 min, followed by chemical treatment in a piranha solution (H2SO4/H2O2, 3:1 v/v) for 5 min. The electrodes were then rinsed with absolute ethanol and finally dried under a flow of nitrogen.
The PAH-coated gold nanoparticles were dispersed in 20 mM phosphate buffer (pH 7.3) containing 5% BSA and 5% Spirulina solution (0.3 g/L). After 1 hour of mixing, a volume of 0.7 µL of this solution was deposited onto the sensitive area of the first pair of IDTs representing the working sensor (figure 1(e)). On the second pair of IDTs representing the reference sensor (figure 1(d)), PAH-coated gold nanoparticles were dispersed in 20 mM phosphate buffer (pH 7.3) containing 20 % BSA and 0.7 µL of this solution was deposited onto the sensitive area of the reference sensor. This configuration allowed differential mode measurements. The sensors were then placed in saturated GA vapours for 30 min. After exposure, the biosensors were dried at room temperature for 30 min and stored at 4˚C before the experiments.
2.8. Biosensor Application to Effluent Samples
Urban sewage and hospital effluents are managed in parallel along two separate lines. They were sampled through automated water sampling at the effluent treatment plant (ETP) entrance and effluent treatment plant output: Hospital Raw Water (HRW), Urban Raw Water (URW), Hospital Treated Water (HTW) and Urban Treated Water (UTW). Three points are tracked on the river: one point upstream of the river (UR), one point immediately downstream of the discharge from the sewage treatment plant (Downstream 1 of the River (DR1)) and one point further along (Downstream 2 of the River (DR2) (figure 2).
3. Results and Discussion
3.1. Cell Immobilization
Electrochemical admittance spectra were recorded for a bare microelectrode (figure 1(b)) and after 12 hours immobilization of Spirulina cells (figure 1(c)). Spectra overlap shows an evolution of complex admittance spectra validating the immobilization process. SEM images revealed the coexistence of several populations of Spirulina cells 0.6 to 1.2 µm in size. The presence of cells which are larger than the filter used could be explained by the pressure applied during filtration that could deform the cells and allow them to pass through the filter.
3.2. Characteristics of Kinetics for Phosphatase and Esterase Activities
The modified electrodes were tested for their sensitivity to both pNPP and AChCl substrates. The t90% response time for AChCl (figure 3(c)) was 80 times faster than t90% recorded for the pNPP (figure 3(a)). This may be due to the high accessibility of the phosphatase sites, compared to esterase sites. These responses include two phases: a first rapid one which corresponds to the external enzymes responses (11 µS) and a second slower phase, corresponding to the response of internal enzymes (figure 3(a), figure 3(c)).
The calibration curve for each enzyme during exposure to various concentrations of pNPP and AChCl were investigated in triplicate and are depicted in figure 4.
As shown in figure 4, the enzymatic activities follow a classical Michaelis-Menten behavior and reach a level corresponding to enzyme saturation at concentrations greater or equal to 1.5 mM pNPP and 3.5 mM AChCl respectively. Mean values and error bars were calculated from three measurements using different biosensors and the relative standard deviation of the sensor did not exceed 10%.
Figure 4(b) presents the calibration curve of the APA with pNPP concentration recorded in Tekaya et al., 2013 [5] , where the Spirulina cells where immobilized directly on electrodes (without gold nanoparticles) and the enzyme activity saturation corresponded to 9 µS at 1.5 mM pNPP. This conductivity increased 3 times when Spirulina cells were immobilized with PAH-coated gold nanoparticles (figure 4(a)). It reaches 30 µS at the same concentration of pNPP (1.5 mM). The use of functionalized gold nanoparticles allows the amplification of the conductivity variation during enzymatic hydrolysis. The same phenomenon was observed with esterase detection from the overlay of both curves (figure 4(d)). Therefore, due to their electrical conductivity, gold nanoparticles can behave as nanoelectrodes, as has already been shown by Nouira et al. [33] in the case of another hydrolase enzyme.
The storage stability of the Spirulina biosensor was monitored for more than 1 month. The biosensor responses as a function of a storage time are shown in figure 5. Each point of the curve corresponded to an arithmetic average of 3 measurements with 3 different biosensors stored in dry conditions at 4˚C (each measurement corresponded to a response to 3.5 mM of AChCl). Experiments show a stability of the conductivity over 25 days. Therefore, the lifetime of the biosensor can be estimated to be more than 25 days.

Figure 2. Scheme representing the sampling sites

Figure 3. Typical real time response of the conductometric transducer based on Spirulina to the pNPP substrate: (a) before incubation (b) after incubation in mixture of parathion-methyl + Cd2+ (10−18 mol/L), and to the AChCl substrate: (a) before incubation (b) after incubation in mixture of parathion-methyl+Cd2+ (10−18 mol/L) for 12 hours (AChCl, 3.5 mM, pNPP, 1.5 mM in 5 mM phosphate buffer at pH 5.2).
3.3. Inhibition Measurements
To evaluate the effects of pollutants on the enzymatic activity of the biosensors, measurements were performed before (dSbefore) and after (dSafter) incubation in 7 concentrations of different mixtures of pesticides/metals: parathion-methyl + Cd2+, parathion-methyl + Hg2+, paraoxon-methyl + Cd2+, paraoxon-methyl + Hg2+, triazine + Cd2+ and triazine + Hg2+. This is because the detection process involves comparing enzyme activity before and after exposure to the inhibitor. The change in sensor response should therefore be attributed to the inhibitor mixture because enzyme activity is still stable and measurable after 25 days. An incubation time of 12 hours has been adopted in this study.
Results were expressed as a percentage of residual activity (Ares) (figure 6) given by the following expression:
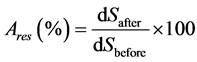 (1)
(1)

Figure 4. Calibration curve of the APA: Variation of conductance versus pNPP concentration ((a) immobilized Spirulina cells in presence of PAH-coated gold nanoparticles (b) Spirulina cells immobilized without gold nanoparticles [5] [6] . Calibration curve of the AChE: Variation of conductance versus AChCl concentration ((c) immobilized Spirulina cells in presence of PAH-coated gold nanoparticles (d) Spirulina cells immobilized without gold nanoparticles. The mean values and error bars have been calculated from 3 experiments with different biosensors, under the same experimental conditions (5 mM phosphate buffer at pH 5.2).&

Figure 5. Evolution of the signal of conductometric biosensors stored at 4˚C in dry conditions.The mean values and error bars have been calculated from 3 experiments with different biosensors, under the same experimental conditions (AChCl, 3.5 mM in 5 mM phosphate buffer at pH 5.2).
Control measurements have been carried out in the same conditions. There was no inhibition phenomenon with an incubated biosensor in pesticide and tested with pNPP, also with an incubated biosensor in metallic cations and tested with AChCl. So, it can be noted that each enzyme was inhibited only by one class of inhibitor.
3.3.1. Effect of Pollutant Mixtures on Biosensor Response Time
Inhibition measurements and pollutants detection experiments were performed at substrates saturation concentration of 1.5 mM pNPP and 3.5 mM AChCl. The t90% response time to pNPP increased after incubation in a mixture of pesticide/metal. An example of a mixture of parathion-methyl + Cd2+ (10−18 mol/L) is shown in Figure 3(b). However, with the same sensor incubated in the same mixture, a remarkable decrease in the t90% electrode response time to AChCl was revealed after incubation (figure 3(d)).
The t90% response time to pNPP recorded before incubation was 12 sec and became 8 min after incubation in a mixture of pesticide/metal. These response times are similar to those recorded with the same biosensor incubated in cadmium, as reported in Tekaya et al. [5] .
The t90% response time to AChCl recorded before incubation in a mixture of pesticide/metal was 75 min and became 6 min after. This is also consistent with the response times recorded with a biosensor incubated with pesticides alone and reported in Tekaya et al., [6] .


Figure 6. Phosphateres and Esteraseres (residual) for 12 h exposure to parathion-methyl + Cd2+, parathion-methyl + Hg2+, paraoxon-methyl + Cd2+ and paraoxon-methyl + Hg2+, triazine + Cd2+ and triazine + Hg2+. The mean values and error bars have been calculated from 3 experiments with different biosensors under the same experimental conditions (pNPP, 1, 5 mM, AChCl, 3.5 mM, in 5 mM phosphate buffer at pH 5.2).
The response in figure 3(b) (after APA inhibition) includes two phases; the first phase is rapid and corresponds to the enzyme response. The second phase is slower and corresponds to a cell rearrangement. In fact, after APA inhibition, the presence of metallic cations prevents the substrate from reaching the phosphatase sites of Spirulina cells easily. This is due to the changes in cell morphology in the presence of heavy metals as shown in figure 7(a), where a change of morphology occurs [5] [6] . This was the first defense mechanism against toxic trace heavy metals. SEM images do not show any modification of the cell morphology after exposure to pesticides at low concentration (figure 7(b)), whereas, at high concentration (10−6 M), a perforation of cell membrane is observed [6] .
No antagonistic effects of the different mixtures could be observed on both enzymatic activities (Phosphatase activity is inhibited only with heavy metals and Esterase activity is inhibited only with pesticides). Besides, interactions of phosphatase with metallic cations are quite different from those of esterase with pesticides (different evolutions in response times before and after incubations in pollutants have been observed). Subsequently, this bi-enzymatic biosensor can be of great interest to discriminate toxic compounds in an unknown sample depending on the inhibited enzymatic activity.

Figure 7. SEM images of Spiruina cell (a) exposed to Cd2+ (10−16 M) (b) exposed to parathionmethyl (10−18 M).
3.3.2. Analytical Performance for Pollutant Mixtures
Both residual Phosphatase and Esterase activities are presented in figure 6. Experiments were carried out after 12 hr exposure to different mixtures of pesticide/metal with equal concentrations going from 10−20 M to 10−06 M (figure 6).
These histograms show that both activities can be inhibited in the presence of parathion-methyl + Cd2+, parathion-methyl + Hg2+, paraoxon-methyl + Cd2+, paraoxon-methyl + Hg2+, triazine + Cd2+, triazine + Hg2+ (figure 6). These observations confirm results obtained with APA measurements alone, reported in Tekaya et al., [5] , and results obtained with AChCl measurements alone, reported in Tekaya et al., [6] and using the same microalga; Arthrospira platensis. These results are summarized in Table 1.
In this study, the quantification limits were lower in comparison with previous studies performed with conductometric biosensors based on Chlorella, with a mixture of paraoxon-methyl + Cd2+ [26] . They are also low compared to acetylcholinesterase pure enzyme biosensors [21] [36] [37] and urease pure enzyme biosensors [21] .
3.4. Residual Activities in Effluent Samples
Enzymatic activities were measured before and after incubation for 12 hours. Residual activities were calculated by providing five different micro-sensors for each sample and are presented in figure 8. Important inhibition of enzymatic activities was revealed in samples HRW, URW, UR, DR1 and DR2 revealing the presence of toxic substances.
After the passage of the effluent throughout the treatment plant, enzyme inhibition decreases due to the effect of water treatment and residual activities (phosphataseres and esteraseres) reached 100% in samples HTW and UTW (figure 8).
Low esterase residual activity is revealed in the Hospital Raw Water (HRW) sample (=10%); this observation could be explained by the presence of some drugs in this effluent that could inhibit esterase activity.
Upstream of urban effluent, there are areas of intensive agriculture thus marking the presence of pesticides in sample taken from URW (figure 8).
Total AChE inhibition in samples DR1 and DR2 is due to the overfull discharged by the municipal wastewater treatment plant in the period of our sampling campaign (figure 8).
The water samples were analyzed by ICP-MS. They show mercury concentrations relatively higher than cadmium concentrations in water samples. A high concentration of mercury was recorded in URW sample (8.9 µg/L) in good agreement with the important inhibition rate of APA recorded with the biosensor (figure 8).
After the passage of the effluent throughout the treatment plant, mercury concentrations decreases going from 1.9 in HRW to 0.8 in HTW and from 8.9 in URW to 0.4 in UTW (table 2). This evolution is consistent with the bi-enzymatic biosensor measurements (figure 8).

Table 1. Quantification limits and IC50 of each pollutant [5] [6] and of each mixture of pollutants.

Figure 8. Phosphataseres and Esteraseres (residual) for 12 h incubation in samples, The mean values and error bars have been calculated from 5 experiments with different biosensors under the same experimental conditions (pNPP, 1.5 mM, AChCl, 3.5 mM, in 5 mM phosphate buffer at pH 5.2).
4. Conclusion
In this work, it is possible to elaborate a bi-enzymatic biosensor with only one bioreceptor based on the inhibition of enzyme activities by toxic compounds. It has been demonstrated for heavy metal ions that the inhibition rates are in a close correlation with the mercury content in the real water sample. The developed biosensor is

Table 2. Heavy metals concentrations determined with ICP-MS methods.
simple and cheap, compared to classical analytical methods and it can provide a rapid scanning of the environment, as an early-warning system.
Acknowledgements
The authors would like to thank CAMPUS-FRANCE for their financial support through PHC Maghreb Project No. 12 MAG 088 and UTIQUE Project No. 13G 1205. The authors thank SIPIBEL program for providing water samples.