Psoas Hematoma Following Lumbar Sympathetic Block in a Patient with Renal and Liver Diseases and Recent Use of Aggrenox ()
1. Introduction
Aspirin/extended release dipyridamole (Aggrenox) is a combination antiplatelet agent that is approved for secondary stroke prevention by the Food and Drug Administration [1] . The incidence of stroke rises progressively with age [2] , so does the chronic pain from degenerative spine and vascular diseases. Facing a growing aging population, interventional pain physicians frequently confront the dilemma of whether to stop or continue antiplatelet/ anticoagulation agents in the periprocedural period. Presently, no guidelines have been written specifically for the management of antiplatelet/anticoagulation therapy around the time of chronic pain-relieving procedures. Common practice has been followed American Society of Regional Anesthesia and Pain Medicine (ASRA) recommendations for the management of antithrombotic therapy when neuraxial and peripheral techniques are performed [3] . To aid future decision making, we present here a case report of psoas hematoma developed after lumbar sympathetic block in a patient with end stage renal failure and liver disease who had limb-threatening ischemia. The patient was treated with Aggrenox until three days before the procedure.
2. Case Report
A 77 year-old white male was referred to the pain clinic for resting left foot pain with gangrene secondary to severe peripheral vascular disease. His past medical history included atherosclerotic coronary heart disease, congestive heart failure, aortic stenosis, atrial fibrillation, end stage renal disease on peritoneal dialysis, and dyslipidemia. Gangrene of the toes of his left foot was noted five months prior to the office visit and his left fourth toe was amputated three weeks prior. Relentless resting pain in the left foot and calf was uncontrolled by oral pain medications. Surgical revascularization or angioplasty was considered but deterred due to concerns of complications. A lumbar sympathetic block (LSB) was considered as a less invasive pain relieving procedure. The patient was taken Aggrenox for secondary stroke prevention. This was prescribed after he developed gastrointestinal bleeding while taking warfarin. Aggrenox was discontinued three days before the LSB. His other medications were Prilosec 20 mg daily, prednisone 20 mg daily, metoprolol 25 mg twice a day, Percocet 7.5/325 every four hours as needed, MiraLAX 17 grams daily, and Nephrocaps daily. Physical examination revealed red, purplish skin discoloration of the left great and second toes. No palpable dorsal pedal pulses were found bilaterally, but femoral pulses were felt. The temperatures before the sympathetic block were left leg 83.7 F and right leg 83.9 F.
After discussing the risks and benefits of LSB, the patient gave consent and was taken to the fluoroscopy suite. He was placed in the prone position with monitors attached to him. The procedure site, 8 cm lateral to the area corresponding to the anterolateral aspect of the upper 1/3 of the L3 vertebra, was prepped as for an aseptic procedure. After the skin and subcutaneous tissue was anesthetized with 1% lidocaine, under fluoroscopy guidance, a 7" bend spinal needle was placed at the anterolateral aspect of the upper 1/3 of the L3 vertebrae. The needle position was confirmed using Isovue dye. After negative aspiration for blood and cerebral spinal fluid, 5 ml of 0.25% bupivacaine was injected. The procedure was completed with one attempt. The patient was transferred to the recovery area for observation. Approximately 15 minutes later, the patient became pale and developed severe back pain, abdominal pain, and nausea with vomiting. The patient’s blood pressure dropped to 70/40 mmHg associated with mottled skin on the left flank. Intravenous fluid was initiated and the patient was admitted to the intensive care unit of the hospital.
The patient’s admission lab data are shown in Table 1. His electrocardiogram revealed nonspecific ST-T wave changes. Abdominal CT scan showed an enlarged left psoas muscle with fluid around it and extending anteriorly into the abdomen (Figure 1). Additional CT images of the patient’s liver revealed inhomogeneous density that was suggestive of cirrhosis with abdominal fluid representing either ascites or blood. Three days after admission, the patient had no left foot pain but persistent back and abdominal pain. His hemoglobin was stable after transfusion and a repeat CT scan showed resolution of the psoas hematoma. Subsequently, vancomycinresistant enterococcus faecium (VRE) peritonitis was diagnosed. Despite aggressive antibiotic therapy and supportive care, the patient’s condition deteriorated progressively and he died a few days later.
3. Discussion
LSB is typically performed to improve lower extremity circulation and relieve pain in patients with peripheral vascular disease and complex regional pain syndrome. The lumbar ganglia aggregate at the anterolateral aspect of upper lumbar vertebral bodies are separated from the somatic nerves by the psoas muscle and the fascia.
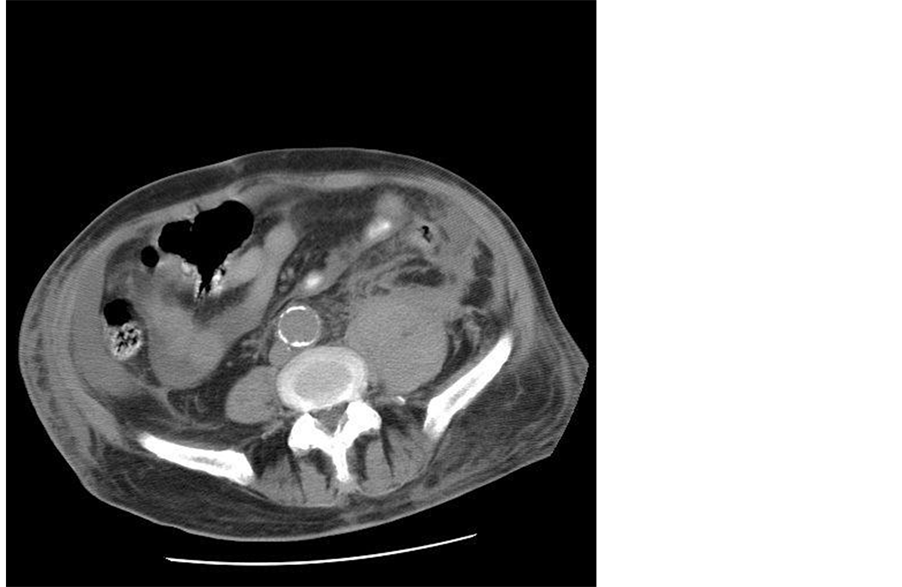
Figure 1. Enlarged left psoas after the lumbar sympathetic block.
The lumbar ganglia are in close proximity to the major vessels [4] . During a LSB, a block needle pierces the psoas fascia and well-perfused muscle [5] . The risk of bleeding from vascular injury is perceivable. Nevertheless, reports of hemorrhagic complication specifically from LSB are scarce. An English language literature search of Pubmed yielded one report of retroperitoneal hemorrhage after LSB in two patients, aged 71 and 79, with severe peripheral arterial disease and critical limb ischemia. The patients had been treated with ticlodipine or clopidogrel [6] .
Anticoagulation by heparin, Coumadin, and thrombolytic agents is considered contraindication to regional anesthesia and nerve blocks. However, antiplatelet agents such as Aggrenox are considered low risk [7] . Each capsule of Aggrenox contains immediate-release aspirin 25 mg and extended-release dipyridamole 200 mg [1] . Aspirin decreases the amount of thromboxane A2, a powerful inducer of platelet aggregation and vasoconstrictor, by irreversibly inhibiting platelet cyclooxygenase. The inhibition of platelet cyclooxygenase can only be reversed by the production of new platelets. Approximately, 30% of platelet cyclooxygenase activity is recovered four days after cessation of aspirin [8] . Dipyridamole increases platelet cyclic-3',5'-adenosine monophosphate (cAMP) levels, resulting in lesser platelet aggregation in response to various stimuli such as platelet activating factor (PAF), collagen, and adenosine diphosphate (ADP). The half-life of extended-release dipyridamole is 13.6 hours [9] . Both drugs are antithrombotic and have a synergistic effect with combined administration [10] . The drug label offers no advice on continuing or stopping Aggrenox around the time of a nerve block [1] . In our case, the medication was ceased three days prior to LSB, and this allowed for partial recovery of platelet function. More often than not, clinicians face the dilemma of continuing or stopping antiplatelet agents around the time of an interventional pain-relieving procedure because the consequences of both hemorrhagic and thrombotic complications are devastating.
Our case also exemplifies the difficultly caring for patients with complex medical co-morbidities in the chronic pain clinic. In addition to the residual effect of Aggrenox, our patient’s renal failure and abnormal liver function were likely contributing factors to his psoas hematoma. The formation of platelet plug is impaired in uremic patients due to a combination of factors that are either intrinsic or extrinsic to platelets [11] . Factors that are intrinsic to platelets are abnormal expression of platelet surface glycoproteins, decreased secretion of platelet granular contents (thromboxane A2, adenosine diphosphate, epinephrine and serotonin), and abnormal platelet cytoskeletal assembly. Factors that are extrinsic to platelets include defective interaction between platelet and vessel wall and between platelet and fibrinogen and anemia. Although our patient did not have frankly de-compensated liver cirrhosis, his low albumin level, marginal prothrombin time, and mildly depressed platelet count demonstrated hepatic dysfunction that likely further negatively impacted his hemostasis. It is also important to note that the patient survived form psoas hemorrhage but died from further complications of VRE peritonitis while in the hospital.
The ASRA guideline published in 2010 entitled “Regional Anesthesia in the Patient Receiving Antithrombotic or Therombolytic Therapy” made no specific recommendation regarding Aggrenox. However, the guideline stresses individualized decisions based on risk and benefit for each patient [3] . Raj et al. [12] proposed a tool which scores the patient-specific and technique-specific bleeding risk factors. The patient-specific factors include history of bleeding, results of coagulation studies, intake of medications known to affect hemostasis, and past medical history relevant to the integrity of the coagulation system. The technique-specific factors examine whether the target is in proximity to significant vascular/neurological structures and/or in a confined space. It also considers needle type (sharp versus blunt) and gauge, number of needle passages, the use of fluoroscopy, the presence of blood during aspiration, and the type of a procedure (single-shot versus continuous infusion). This allows physicians to quickly identify potential bleeding problems.
4. Conclusion
In conclusion, LSB is a commonly used technique in pain management for patients with severe peripheral arterial disease who are not candidates for surgery or angioplasty. This patient population usually has multiple medical co-morbidities and has high risk for procedural complications. The occurrence of significant bleeding is rare based on published literature, despite the fact that the incidence of vascular puncture is certainly more common than reported. The pain practitioner must weigh the risks and benefits carefully and keep in mind the risks associated with the use of antiplatelet agents as well as those from discontinuation of such agents. Nerve blocks provide short term pain relief in general and long term pain control is achieved by repeated procedures. Complications from multiple procedures are conceivably higher than that from one time intervention. Clear communication must be maintained between the interventional pain physician and the patient.
NOTES
*Corresponding author. Current Address: Department of Neurology, Veterans Affairs Medical Center, Washington DC, USA.