Synthesis of Poly(APP-co-EGDMA) Particles Using Monomers Derived from Cashew Nut Shell Liquid for the Removal of Cr(III) from Aqueous Solutions ()
1. Introduction
Environmental pollution by heavy metals is a widespread problem nowadays due to increased anthropogenic activities. In most cases, the pollution load ends into water bodies thus threatening not only aquatic organisms, but also the quality of water for domestic uses. Pollution from heavy metals is one of the most serious environmental problems due to the fact that they bio-accumulate causing adverse effects to living organisms [1]. In view of this, their removal from industrial discharged effluents becomes an absolute necessity.
Chromium is one of the heavy metals known to be toxic to humans and aquatic life. Various industrial effluents from pigments, electroplating, dying, canning, textile, leather tanning, paint and steel industries contain substantial amounts of chromium. In general, these industrial effluents contain both Cr(III) and Cr(VI) ions. However, it is well established that Cr(III) is not as toxic as Cr(VI). Yet under suitable conditions, Cr(III) can readily be oxidized to Cr(VI) [2], thus underlying the importance of restricting its accumulation in the environment. Furthermore, excessive buildup of Cr(III) ions can affect the ecology of the environment as well as inhibiting various enzyme systems in living organisms [3,4]. Long term exposure to Cr(III) is also known to cause cancer and allergic skin reactions [5].
The importance of minimizing the amount of Cr(III) ions in industrial effluents and other sources has led to the development of a number of removal methods. The commonly used methods for removing such metal ions from aqueous waste include precipitation, lime coagulation, semi-sedimentation, electro-dialysis, chemical reaction, biological processes, ion exchange, filtration, reverse osmosis, solvent extraction and adsorption [6].
Adsorption process is the most common and is widely reported in the literature. Thus a number of adsorbents ranging from inorganic to organic polymers have been investigated. Examples of studied organic polymers include polystyrene [7], polyaniline [8,9], polypyrrole [10], polypropylcalix [6] arene [11], polyacrylicester [12], poly (MMA-MAGA) [13], poly(GMA-co-EDGMA)-en [14] and polystyrenedivinylbenzene [15]. However, these polymers are prepared from commercial monomers, which are often time-consuming, expensive and not renewable. In an effort to use renewable monomers, we have attempted the synthesis of co-polymer particles using monomers derived from CNSL, a by-product of the processing of cashew (Anacardium occidentale L.) nuts. The co-polymer particles were investigated as adsorbents for the removal of Cr(III) ions from aqueous solutions.
2. Experimental
2.1. Chemicals and Materials
Ethylene glycol dimethacrylate (EGDMA) 98% and sodium nitrate (Aldrich, UK) were used as received. The stock solution containing 1000 mg/L Cr(III) was prepared by dissolving a known quantity of chromium nitrate nonahydrate (Lobachemie, Mumbai India) in distilled water. This stock solution was diluted as required to obtain the working solutions containing 0.16 to 20.00 mg/L Cr(III). All other chemicals were of analytical reagent grade and were used as received. CNSL was extracted from cashew nut shells obtained from Mtwara region in Tanzania. Cashew nut shell pieces (725 g) were soaked in cyclohexane (500 mL) for 3 days; thereafter the shells were sieved out, and the solution filtered and concentrated under reduced pressure using a rotary evaporator at 40˚C. A brownish CNSL product (228 g, 31%) was obtained. Thereafter, anacardic acid (1) (Scheme 1) was obtained from the CNSL via calcium anacardate procedure as reported elsewhere [16], with a few operational modifications.

Scheme 1. Different stages in the synthesis of aminopentadecylphenols.
2.2. Synthesis of Amino-Pentadecylphenols from Anacardic Acid
The anarcadic acid (50.0 g) was hydrogenated in the presence of palladium catalyst to give 2-hydroxy-6-pentadecyl-benzoic acid (2) (10.60 g, 21.2%) as off-white crystalline material. IR n (-COOH) = 3500 - 3100 cm−1, n (Ar-OH) = 3400 - 2400 cm−1, n (-COOH) = 1631 cm−1, n (Ar-H) = 3009 cm−1, n (aliphatic C-Hst) = 2914 cm−1 and 2848 cm−1, n (aromatic C=C) = 1444 cm−1 and n (Ar-OH and -COOH) = 1310 cm−1. The 1H-NMR (500 MHz, CDCl3) showed the substitution pattern of the benzene ring leading to the structure of the product. The presence of three aromatic protons observed at δ 6.785 (d), 6.879 (d), and 7.362 (t) indicates that the product is a tri-substituted benzene. The alkyl protons were observed at δ 0.862 - 2.983 (m). The phenolic and carboxyl protons were assigned at δ 5.026 (m, mixed) and 11.026 (s), respectively. The 2-hydroxy-6-pentadecyl-benzoic acid (2) (9.17 g) was then decarboxylated to 3-pentadecylphenol (3) by heating at 200˚C for 5 h. The product (7.85 g, 98%) was obtained as a dark brown liquid that solidified after cooling to room temperature. The IR spectrum shows similar features as for (2), possibly because absorption bands for -COOH and aromatic -OH groups do overlap. Nonetheless, the 1H-NMR (500 MHz, CDCl3) spectrum displays similar features to that of 2-hydroxy-6-pentadecyl-benzoic except for the absence of exchangeable ArCOOH at δ 11 ppm and the presence of a broad peak (ArOH) at around δ 4.7 - 5.0 ppm.
The 3-pentadecylphenol (3) (7.85 g) in dichloromethane was nitrated heterogeneously using a mixture of sodium nitrate (10.22 g), sodium hydrogen sulfate monohydrate (16.55 g) in wet silicon dioxide (50% w/w). Work-up of the reaction afforded yellow precipitates that solidified upon addition of ice water. Re-crystallization of this material from methanol afforded yellow solid (2.70 g). FT-IR spectrum (Figure 1) showed the appearance of new distinctive peaks around 1200 cm−1 and 1650 cm−1 that indicate the presence of nitro group.
1H-NMR (500 MHz, CDCl3) (Figure 2) δ. 0.9 - 3.0 ppm are characteristic aliphatic proton peaks; singlet

Figure 1. IR spectrum for a mixture of nitro-pentadecylphenols.
peaks at around δ 7.3 and 9.0 ppm attributed to aromatic protons. Since these peaks are singlets, it implies that the signals are from nonadjacent aromatic hydrogen atoms. It can therefore be predicted that the nitration process resulted to a mixture of diand tri-nitro compounds, i.e., 5-pentadecyl-2,4-dinitrophenol (with two aromatic protons) and 3-pentadecyl-2,4,6-trinitrophenol (with one aromatic proton). The trinitro product, 3-pentadecyl-2,4,6- trinitrophenol, displays a 1H NMR peak at δ 9.0 ppm for the aromatic proton between two nitro groups, i.e., more de-shielded. On the other hand, the dinitro product, 5-pentadecyl-2,4-dinitrophenol, displays 1H NMR peaks for the aromatic proton between phenol and aliphatic groups (at δ 7.3, less deshielded), and between two nitro groups (at 9.0 ppm, more deshielded). A weak and broad peak at around δ 11.2 ppm is associated with phenolic -OH proton.
Reduction of the mixture of nitropentadecyl phenols (4) (5.0 g) using tin chloride and hydrochloric acid gave compound (5) as black solid. Characterization of this mixture of compounds using IR (Figure 3) showed disappearance of peaks around 1200 cm−1 and 1600 cm−1 (-NO2) and appearance of distinctive peaks at 3550 - 3110 cm−1 showing axial deformation of N-H groups, 1374 cm−1 a strong band corresponding to aromatic amines, at around 1125 cm−1 from axial deformation of the C-N group, 1572 cm−1 (N-H) indicating presence of amines.

Figure 2. 1H-NMR (500 MHz, CDCl3) spectrum for a mixture of nitro-pentadecylphenols.

Figure 3. IR-spectrum for a mixture of amino-pentadecylphenols.
The 1H-NMR (500 MHz, CDCl3) (Figure 4) δ 0.9 - 3.0 ppm = aliphatic protons in the same manner as for compounds (2) and (3). In addition, a singlet proton peak at δ 7.2 associated with mixed chemical exchangeable protons of the aromatic amine -NH and phenolic -OH.
2.3. Co-Polymerization of APP and EGDMA
A mixture of APP components was used for polymerization without an attempts separation. Polymerization of APP and EGDMA was done through aza-Michael addition starting with 1.05 g of APP and 5 mL of EGDMA. Without initiator, polymerization reaction was performed by setting the vessel to the refluxing mantle. The refluxing was done at a temperature of 180˚C for eight hours. The product was filtered and the residues washed with excess ethanol followed by dichloromethane. 1.65 g of polymer was obtained. The polymer particles were characterized by FT-IR and Scanning Electron Microscopy (SEM).
2.4. Characterization of the Co-Polymer Particles
Scanning electron microscopy (SEM) was used to study the surface morphologies and grain size of the co-polymer materials. Perkin-Elmer 2000 FTIR spectrophotometer was employed in the determination of the surface functional groups of the co-polymer particles. The spectra were recorded from 4000 to 600 cm−1. Furthermore, the amount of amino functional groups incorporated onto the co-polymer surfaces were determined by an acid titration method. The amount of surface bound species is equivalent to the amount of HCl acid consumed.
2.5. Cr(III) Adsorption Experiments
Chromium (III) adsorption isotherms were obtained using a batch equilibration procedure. Duplicate 0.015 g polymer particles were equilibrated for 4 h with 100 ml of aqueous solutions of Cr(III) with concentrations ranging from 0.16 to 20.00 mg/L. The pH values of the test solutions were maintained at pH 6.0 by adding HCl or NaOH solutions, a pH that was well below the precipitation level of chromium (pH > 8) [17]. After equilibration the content of each flask were filtered through a Whatman No. 50 filter paper, and the filtrate analyzed for Cr(III) ion using atomic absorption spectrophotometer (novAA 400, Analytic Jena AG). The Cr(III) ions were removed from the adsorbents by washing with dilute HCl followed by distilled water. After filtration and drying, the recovered adsorbents were reused in a fresh batch as explained earlier.

Figure 4. 1H-NMR (500 MHz, CDCl3) spectrum for a mixture of amino-pentadecylphenols.
3. Results and Discussion
3.1. Synthesis and Characterization of Poly(APP-co-EGDMA) Particles
Figure 5 shows FTIR spectrum of the poly(APP-coEGDMA) particles. For the synthesized polymer, important peaks include those at around 1125 - 1572 cm−1 which signify the presence of amines, as well as a broad band around 3100 cm−1, which is attributed to phenolic characteristics.
As determined by acid titration method, the loading of -NH groups on the surface of the synthesized poly(APPco-EGDMA) particles was found to be 46 mmol per g of dry polymer. This amount is higher than most amino groups loadings found in functionalized mesoporous silicas. For instance, the Santa Barbara Amorphous material (SBA-15) containing one, two and three amino functional groups have amine loading ranging from 0.9 to 6.0 mmol/g [18]. The reason for this abnormally high loading can be attributed to the flexibility of the co-polymer, which renders the accessibility of the amine groups to neutralization reaction.
Figure 6 shows the morphology of the prepared polymer particles. It is apparent from this figure that the poly(APP-co-EGDMA) particles obtained were spherical and of small size. Although these particles were found to have sizes ranging from 0.5 - 2.5 µm, about 50% of the particles had sizes in the range 1 - 1.5 µm, thus signifying a narrow size distribution (Figure 7).
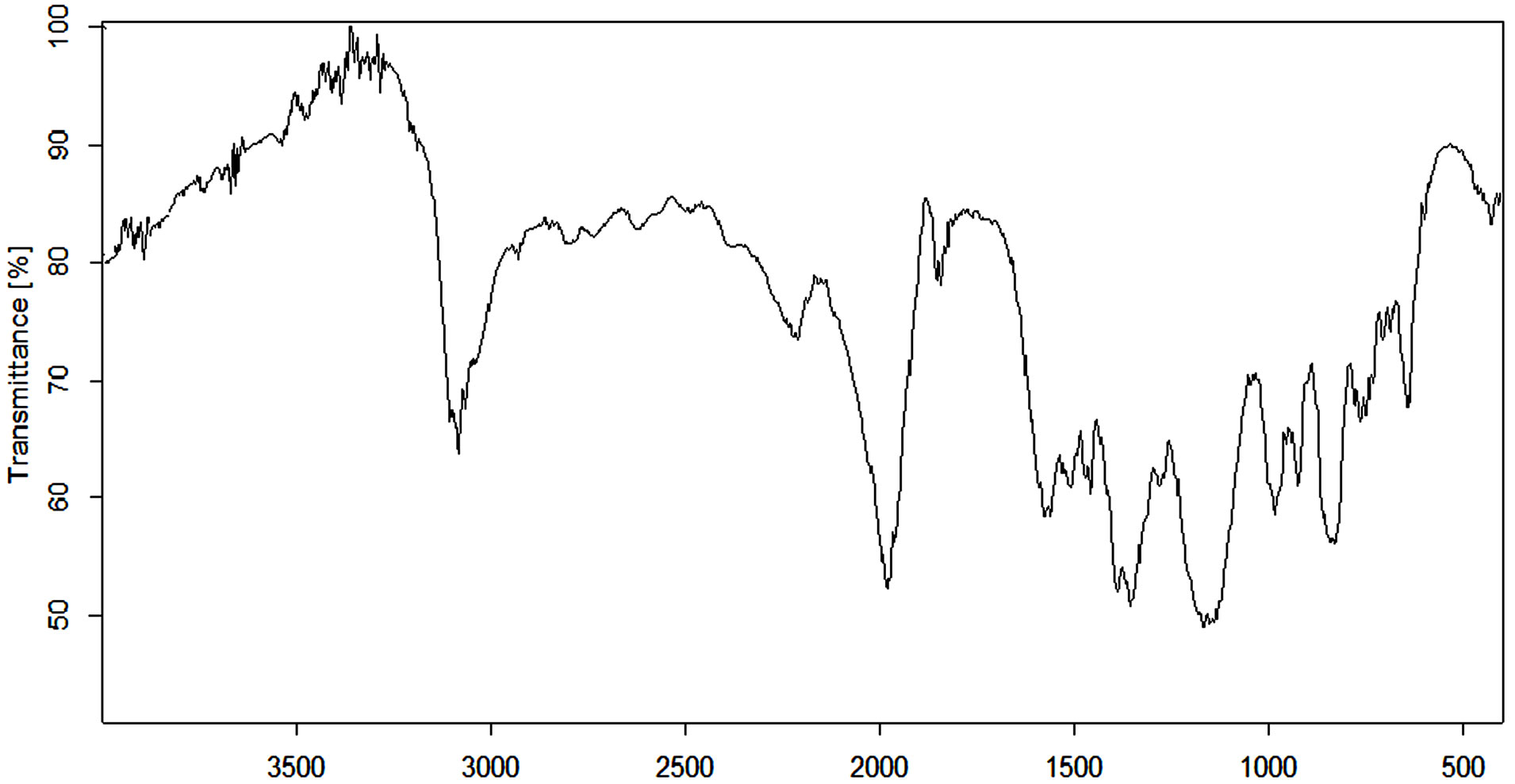
Figure 5. FTIR spectrum of the poly(APP-co-EGDMA) particles.

Figure 6. A SEM micrograph of poly(APP-co-EGDM) particles.

Figure 7. A histogram showing particle size distribution of the poly(APP-co-EGDM) particles.
3.2. Adsorption Isotherm Studies
Cr(III) ions adsorption on the synthesized poly(APP-coEGDM) particles at different ion concentrations was investigated. The adsorption test was done by batch method as explained elsewhere [19]. Results for the Cr(III) adsorption are shown in Figure 8, with removal efficiencies calculated using Equation (1).
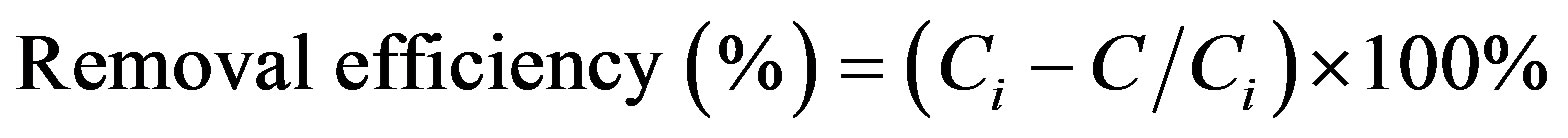 (1)
(1)
where, Ci and C represent the initial and equilibrium concentrations (mg/L), respectively. As seen from this figure, the percentage Cr(III) ion adsorption decreased from 75% to 11% with the increase in initial concentrations of Cr(III) ions from 0.16 to 20.00 mg/L. This is the indication that the Cr(III) ions uptake is predominantly dependent on initial Cr(III) ion concentration. At low concentrations, Cr(III) ions are adsorbed at specific sites, but with increasing Cr(III) ion concentrations the sites tend to be saturated very fast.
Generally at the lower Cr(III) ion concentrations, the removal percentage was higher due to a larger surface area of the polymer being available for the adsorption of Cr(III) ions. When the concentration of the Cr(III) ions became higher, the removal percentage was lower because the available sites for the adsorption became less. At a higher initial Cr(III) ion concentration, the ratio of initial number of moles of Cr(III) ion to the available adsorption surface area was high and as a result adsorption percentage was less.
To examine the relationship in distribution between adsorbed (Cads) and equilibrium Cr(III) ion concentration (Ceq), adsorption isotherm models are widely employed for fitting the data. The most widely used isotherm models are the Langmuir and Freundlich equations. Whereas the Langmuir model assumes that the uptake of metal ions occurs on a homogenous surface by monolayer adsorption without any interaction between adsorbed ions, the Freundlich model is more empirical and it assumes a heterogeneous adsorption due to the diversity of the ad-
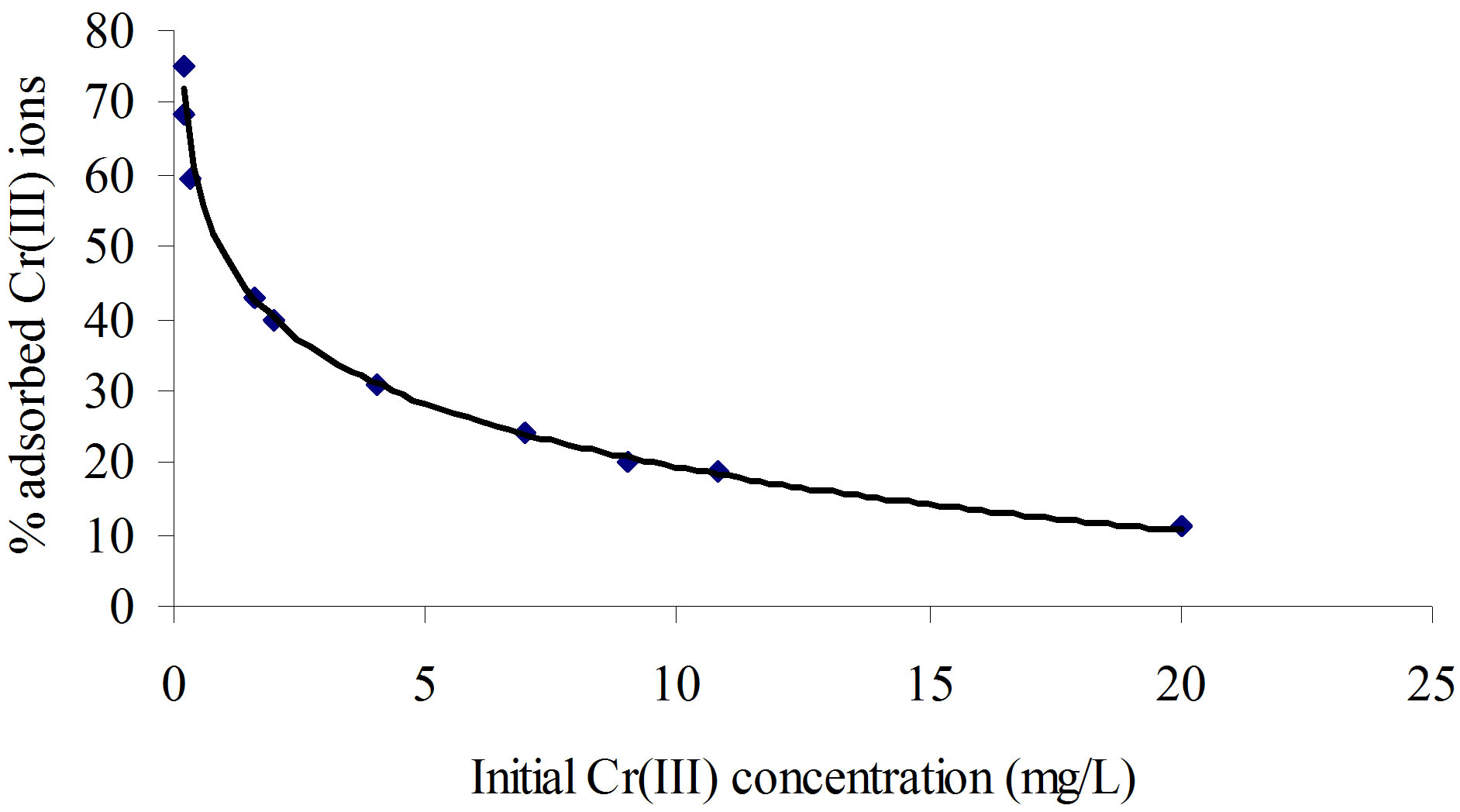
Figure 8. A plot of initial concentration of Cr+3 ion as a function of percentage Cr+3 ion adsorbed by 0.15 g of poly(APP-co-EGDMA) particles per litre of solution containing varying Cr+3 ion concentration.
sorption sites or the diverse nature of the metal ions adsorbed, free or hydrolyzed species. To get the equilibrium data, initial Cr(III) ions concentration were varied while the adsorbent mass in each sample was kept constant. A graph of Cr(III) ions adsorbed on poly(APP-coEGDMA) particles, Cads (mg/g), was then plotted as a function of the equilibrium solute concentration Ceq (mg/L) (Figure 9). As seen from the figure, initially the isotherm curve is characterized by a region which is concave to the concentration axis, and then it reaches a plateau where there is no more adsorption. The obtained data were correlated with a linearised form of the Langmuir Equation (2),
 (2)
(2)
where, qm is the maximum adsorption capacity (mg/g) and Ka is the equilibrium constant related to the energy of adsorption (L/mg).
A typical plot is given in Figure 10. As seen from the figure, the data seem to be well described by the linear form of the Langmuir equation with R2 value of 0.99. The high degree of the correlation coefficient (R2) for the linearized Langmuir relationship suggests that a single surface reaction with constant activation energy is the predominant sorption step [7]. This is also in line with the gradual decrease in the percentage of Cr(III) ions removed from the solution as the initial concentration was increased (Figure 8). The values of the Langmuir adsorption parameters i.e. qm and Ka deduced from the slopes and intercepts of the plot are 16 mg/g and 0.6 L/mg, respectively.
The Freundlich adsorption model was also applied to the adsorption of the Cr(III) ions. A typical plot from the linearised Freundlich Equation (3) is given in Figure 11.
 (3)
(3)

Figure 9. Cr(III) ions adsorption isotherm on poly(APPco-EGDMA) particles.

Figure 10. A Langmuir plot for the adsorption of Cr(III) ions on poly(APP-co-EGDMA) particles.

Figure 11. A Freundlich plot for the adsorption of Cr(III) ions on poly(TAPP-co-EGDMA) particles.
Results indicated that the value of R2 is about 0.95. The relatively low correlation of the Freundlich equation over the Langmuir equation proves further that the adsorption process is mainly monolayer. The coefficient k is a parameter that reflects the amount of the active adsorption site and n characteristic coefficient related to energy or intensity of adsorption. Values of n between 2 and 10 indicate a good adsorption [24]. The values of n and k obtained from this study were 2.6 and 5.4, respectively.
3.3. Polymer Reusability
After washing the polymer with dilute acid followed by distilled water, the polymer particles were dried at 50˚C and then allowed to cool to room temperature. The materials were then used in a fresh adsorption experiment. Results indicated that the reused materials have an adsorption maxima of 13 mg Cr(III) per g, which is over 80% efficiency as compared to the freshly prepared poly(APPco-EGDMA) particles.
3.4. Comparison of Cr(III) Removal with Different Adsorbents Reported in the Literature
The adsorption capacity of the poly(APP-co-EGDMA) adsorbent for the removal of Cr(III) was compared with other adsorbents reported in the literature, and the values of the adsorption capacities are presented in Table 1. As it can be seen from the table, the sorption capacity of poly(APP-co-EGDMA) is comparable with the reported ones. The variations in adsorption capacity that are observed could be attributed to the characteristics of the individual adsorbent, the extent of surface/surface modification, the pH, and the initial concentration of the adsorbate [25].
4. Conclusion
Poly(APP-co-EGDMA) particles were successfully synthesized by a copolymerization of a mixture of amino pentadecylphenols (derived from CNSL) and ethylene glycol dimethacrylate. Characterization of the poly(APPco-EGDMA) particles indicated that they are spherical in nature, with grain size ranging from 0.5 - 2.5 µm. The particles had an amino group loading of 46 mmol/g. The prepared co-polymer particles were found to have a maximum adsorption capacity for Cr(III) ions of 16 mg per g of dry polymer. The spent polymer particles were recoverable and reusable.

Table 1. A comparison of Cr(III) removal with different adsorbents reported in the literature.
Acknowledgements
The authors would like to thank the University of Dar es Salaam and St. John’s University of Tanzania for logistical and financial support.