Aqueous-Phase, Palladium-Catalyzed Suzuki Reactions under Mild Conditions ()
1. Introduction
Palladium-catalyzed Suzuki cross-coupling reaction, widely used synthetic methodology, is one of the most powerful methods for the formation of C-C and C-heteroatom bonds [1-3]. Generally, triphenylphosphines are the most commonly used ligands to achieve reasonable levels of activity [4]. However, phosphine ligands like triarylphosphines typically show high toxicant toward the environment, and are difficult to be separated from organic products. Recently, it has been reported that ionic liquids (ILs) have been used as media in the cross-coupling reaction to serve as the mobile supports of Pd catalysts due to environmental, economic, and safety concerns [5,6]. Although ionic liquids have been developed as phosphine-free ligands, most of these ligands are based on ordinary ionic liquids without functionalized groups, therefore coordinating abilities of these phosphine-free catalytic systems are often very poor [7,8]. It means that catalytic activity using these ionic liquids as ligands is lower compared with phosphine-containing ligands. Thus, the development of more effective methodologies for improving the ligation ability of ionic liquids has a longstanding interest in organic synthesis. A feasible method to achieve such improvement could be the introduction of functionalized groups into ILs. For this purpose, the ionic liquids with electron-donating groups, for example, imidazole [9], nitrile group [10], pyridyl moiety [11], pyrazolyl [12] and so on [13,14], have been investigated as phosphine-free ligands.
Our research group has also had a long-standing interest in Pd-catalyzed cross-coupling reactions utilizing functionalized-ionic liquids as phosphine-free ligands. Recently we disclosed a catalyst system, comprised of diol-functionalized ILs, 2,2-bis((1-methylimidazolium)- methyl) propane-1,3-diol hexafluorophosphate and 1-(2, 3-dihydroxypropyl)-3-methylimidazolium hexaflu-orophosphate, which showed excellent reactivity for Heck reaction of aryl iodides/bromide and acrylates owing to available multi-coordinating sites in these ligands [15- 18]. In light of these advances, we envisioned that if the use of diol-functionalized ILs could allow Suzuki reaction of aryl iodides/bromides to reach the same efficiencies as Heck reaction. Therefore, in considering further study, we intend to extend the scope of diol-functionalized ILs to explore the reactivity of the Pd-catalysed Suzuki reactions of aryl boronic acids with aryl iodides/bromides/chlorides. In this context, we are interested in applying water-soluble, diol-functionalized ILs with different cations as phosphine-free ligands (Scheme 1) in an effort to achieve similar levels of activity that have been achieved in phosphine-containing ligands. It is also noting that the potential of water as a medium for the synthesis has been discussed because of the economy and safety of using water as a solvent.
2. Results and Discussion
We initially focused on the Pd-catalyzed coupling reaction of phenyl boronic acid with phenyl iodide in the presence of diol-functionalized IL, 1. The previous studies [19] observed that the choice of solvent was very important for the reaction. Water represents one of the most economically and environmentally viable options. However, catalysts gave low activities when the reaction was carried out in neat water due to the low solubility of phenyl iodide in water. Therefore, 1:1 CH3CN/H2O was chosen as the solvent in the reactions. In all the reactions, room temperature was adopted with K2CO3 as a base in the presence of 2.0 mol% PdCl2 as well as 4.0 mol% diol-functionalized IL ligands.
At the outset of our studies, we found that ligand 1, which was highly efficient for Heck reaction, gave only moderate yield of 78% for Suzuki reaction with Pd precipitation (Table 1, Entry 1). With careful control of the conditions, it was discovered that method of operating the reaction is very important for the reaction, as low yield was obtained when all substrates was added in CH3CN/H2O. Notably, a significant increase in yield of 96% occurred when the mixture of phenyl iodide and phenyl boronic acid in CH3CN was added into the aqueous PdCl2-1, and finally K2CO3 was added (Table 1, Entry 2). Then we examined the efficiency of using PdCl2 in combination with ligands 2 - 9 under the same conditions. Table 1 demonstrates that pyridinium ionic liquid ligands, 7 and 8, gave only moderate yields for C-C cross-coupling (Table 1, Entries 8 and 9). Similarly, 6 with 1,2-dimethylimidazolium cation and 9 comprising of piperidinium also give disappointing results (Table 1, Entries 7, 10). On the other hand, other compounds bearing imidazolium cations such as 2 - 5 are active for this cross-coupling reaction (Table 1, Entries 3-6). Moreover, 2,2-bis((1-hexylimidazolium)methyl) propane-1,3- diol hexafluorophosphate 4 easily appears to be the most efficient of the ligand tried (Table 1, Entry 5), giving the coupling product in 98% isolated yields at 30˚C within 30 min.
In next set of experiments, besides K2CO3, we examined the influence of altering the bases such as Na2CO3, NaHCO3, NMP, pyridine, Et3N, and (CH3)3CNH2 in the reactions (Table 1, Entries 11-16). This confirms that K2CO3 was the best of the base tried for both yield and selectivity. The optimum loading of Pd catalyst for the reaction was found to be 2.0 mol% along with 4.0 mol%

Scheme 1 . Ionic liquid-supported diols.

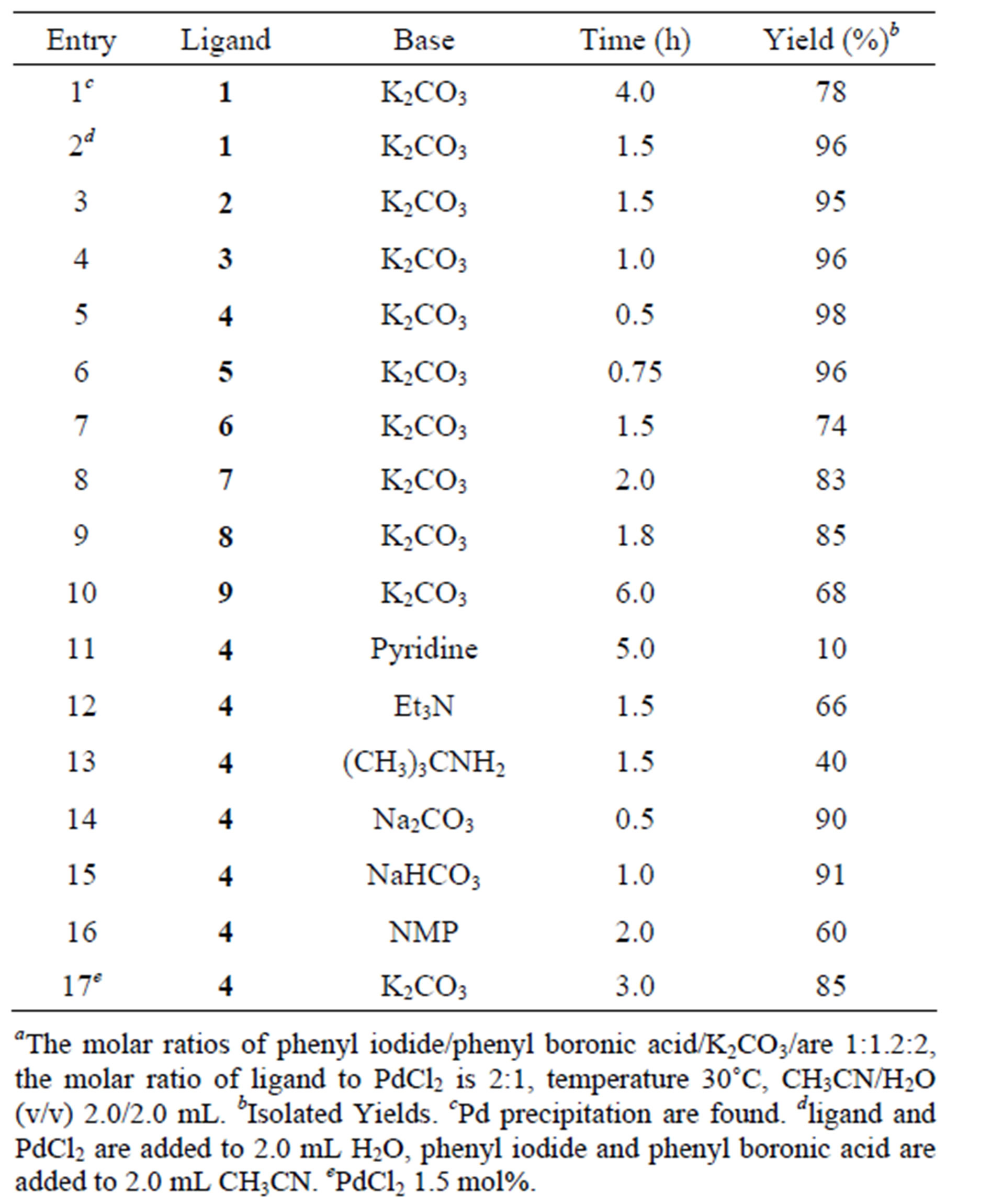
Table 1. The Suzuki reaction of phenyl boronic acid with phenyl iodidea.
of ligand (Table 1, Entry 5). However, a significant decrease in the product yield of (98% to 85%) and increase reaction time (0.5 h to 3.0 h) was observed in the reaction when the catalyst loading was decreased from 2.0 mol% to 1.5 mol%, (Table 1, Entry 17).
With a set of optimized conditions in hand, we explored the breadth of application of this new method to a broad range of targets, including aryl boronic acids and aryl iodides/bromides/chlorides substituted by electronwithdrawing and electron-donating groups (Table 2). The PdCl2-4 system was found to effectively arylate aryl boronic acids with electron-rich and electron-poor aryl


Table 2. Suzuki reactions of haloarenes with aryl boronic acidsa.
iodides/bromides and gave the desired coupled products in excellent/good/moderate yields in the presence of optimized conditions. However, an attempt to couple omethyl or o-trifluoromethyl iodobenzene with phenyl/pmethyl phenyl boronic acid gave a lower yield, probably owing to steric hindrance (Table 2, Entries 4, 5, 10, and 11). Introduction of an electron-releasing methoxy group on aryl boronic acids resulted in moderate yields of the products (Table 2, Entries 7, 8), which is not according with the previous report [19]. Unfortunately, however, a lower yield was obtained with aryl chlorides (Table 2, Entries 15 and 16) probably due to poorer activity of chlorides.
It is all known that using water as a solvent is attractive because of the economy and safety. To further expand substrate scope of this methodology, neat water may be used as the solvent for the Suzuki coupling reaction in the presence of the PdCl2-4 system (Table 2, Entries 17 - 20). The hydrophilic substrates 4-nitrobromobenzene as a substrate was investigated because the former study has shown the importance of water solubility to the conversion. It was pleased that good yields of coupled products (78% - 99%) were obtained in neat water. Notably, reactions appeared to be more sensitive to substituents on aryl boronic acid, as both electron-withdrawing and electron-donating groups gave lower yields in this system.
It is highly desirable that the catalysts can be recovered and reused from the perspective of green chemistry. In case of the reaction with PdCl2-4 we observed that the product had differential solubility from the catalyst-IL in Et2O. This enables us to extract the product by extraction of the reaction mixture with a minimal amount of Et2O whereupon the catalyst-IL was retained in the reaction flask and could be reused. The catalyst PdCl2-4 could be removed from the reaction mixture by extraction and reused at least four times without significant loss of activity and selectivity. After the fourth cycle, it was necessary to separate the aqueous catalyst solution from a large amount of precipitated salts. This extra level of manipulation may have contributed to the significant loss of activity in cycle 5 (Table 3).
3. Conclusion
In summary, we have disclosed that diol-functionalized IL 4, a phosphine-free ligand, can efficiently promote the Suzuki reaction of aryl boronic acids with aryl halides using PdCl2 as a catalyst under significantly milder conditions. It provides a green ligand for selection of palladium-catalyzed arylation for practical use. The diol-functionalized IL ligands allow coupling reactions of both activated and deactivated aryl iodides/bromides under mild conditions and is tolerant of some degree of steric bulk on the aryl iodides/bromide. Hydrophobic reaction products from the Suzuki reactions can be easily isolated by using ethyl ether. Furthermore, the use of water in combination with renewable catalyst represents an environmentally sustainable approach to the coupling of hydrophilic aryl bromides.
4. Experimental
4.1. General Procedure for Synthesis of Ionic Liquid Ligands
A mixture of 2,2-bis-(bromomethyl)-propane-1,3-diol (1 mmol) and 1-alkylimidazole/1-alkylpyridine/1,2-dime-

Table 3. Stability study for PdCl2-4 catalysta.
thylimidazole/1-methyl piperidine (3 mmol) was stirred vigorously at 150˚C for 8 h. After cooling to room temperature, the crude product was washed with acetone. The resulting solid collected by filtration was treated with water (5 mL) as well as KPF6 (2 mmol) and the reaction mixture was stirred at room temperature for 1 h. After filtration, the white solid was washed with ethanol and dried in vacuo to give the desired product.
4.2. The NMR and FT-IR Data for the Products
2,2-bis((1-methylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (1). White solid (0.47 g, 84%); FT-IR (KBr) ν: 3604, 3165, 3122, 2977, 2913, 1588, 1577, 1448, 1425, 1301, 1173, 1014, 846 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 3.10 (d, J = 4 Hz, 4H, OH-CH2), 3.90 (s, 6H, CH3), 4.25 (s, 4H, N-CH2), 5.25 (t, J1 = 5 Hz, J2 = 4 Hz, 2H, OH), 7.70 (s, 2H, NCH), 7.80 (s, 2H, NCH), 9.15 (s, 2H, N (H) CN).
2,2-bis((1-ethylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (2). White solid (0.42 g, 72%); FT-IR (KBr) ν: 3620, 3590, 3180, 1560, 1460, 1350, 1160, 1060, 839 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 1.42 (t, 6H, J = 5 Hz, CH2-CH3), 3.11 (d, 4H, J = 5 Hz, CH2-OH), 4.20 (q, 4H, N-CH2-CH3), 4.22 (s, 4H, N-CH2), 5.32 (t, 2H, J = 5 Hz, OH), 7.62 (s, 2H, NCH), 7.84 (s, 2H, NCH), 9.03 (s, 2H, N (H) CN).
2,2-bis((1-butylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (3). White solid (0.46 g, 72%); FT-IR (KBr) ν: 3600, 3160, 2970, 2930, 1570, 1450, 1170, 1020, 845 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 0.90 (t, 6H, J = 5 Hz, (CH2)3-CH3), 1.25 (m, 4H, (CH2)2CH2-CH3), 1.77 (m, 4H, CH2-CH2-CH2-CH3), 3.11 (d, 4H, J = 5 Hz, CH2-OH), 4.18 (t, 4H, J = 5 Hz, N-CH2-(CH2)2 CH3), 4.24 (s, 4H, N-CH2), 5.31 (t, 2H, J = 5 Hz, OH), 7.64 (s, 2H, NCH), 7.84 (s, 2H, NCH), 9.05 (s, 2H, N (H) CN).
2,2-bis((1-hexylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (4). White solid (0.45 g, 65%); FT-IR (KBr) ν: 3610, 3180, 3060, 2960, 2930, 2860, 1570, 1460, 1170, 1010, 839 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 1.55 (t, 6H, J = 5 Hz, (CH2)5-CH3), 1.96 (m, 12H, (CH2)2-(CH2)3-CH3), 3.80 (d, 4H, J = 2.5 Hz, CH2-OH), 4.02 (m, 4H, CH2-CH2-(CH2)3-CH3), 4.88 (t, 4H, J = 5 Hz, N-CH2-(CH2)4-CH3), 4.96 (s, 4H, N-CH2), 6.02 (t, 2H, J = 5 Hz, OH), 8.36 (s, 2H, NCH), 8.54 (s, 2H, NCH), 9.82 (s, 2H, N (H) CN).
2,2-bis((1-octylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (5). White solid (0.58 g, 77%); FT-IR (KBr) ν: 3610, 3170, 2920, 2850, 1570, 1460, 1180, 1010, 837 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: (t, 6H, J = 5 Hz, (CH2)7-CH3), 1.24 (m, 20H, (CH2)2- (CH2)5-CH3), 1.79 (m, 4H, CH2-CH2-(CH2)5-CH3), 3.09 (d, 4H, J = 5 Hz, CH2-OH), 4.17 (t, 4H, J = 5 Hz, N-CH2-(CH2)6-CH3), 4.24 (s, 4H, N-CH2), 5.31 (t, 2H, J = 5 Hz, OH), 7.63 (s, 2H, NCH), 7.84 (s, 2H, NCH), 9.04 (s, 2H, N (H) CN).
2,2-bis((1,2-dimethylimidazolium)methyl) propane-1, 3-diol hexafluorophosphate (6). White solid (0.40 g, 68%); FT-IR (KBr) ν: 3605, 3160, 2980, 1590, 1570, 1450, 1430, 1300, 1171, 1015, 840 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 2.64 (s, 6H, CH3), 3.11 (d, 4H, J = 1.5 Hz, OH-CH2), 3.76 (s, 6H, N-CH3), 4.19 (s, 4H, N-CH2), 5.41 (s, 2H, OH), 7.50 (s, 2H, NCH), 7.68 (s, 2H, NCH).
2,2-bis((1-pyridinium)methyl) propane-1,3-diol hexafluorophosphate (7). White solid (0.39 g, 71%); FT-IR (KBr) ν: 3608, 3060, 2880, 1592, 1575, 1455, 1432, 1301, 1170, 1015, 837 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 3.16 (s, 4H, OH-CH2), 4.78 (s, 4H, N-CH2), 5.58 (s, 2H, OH), 8.19 (m, 4H, m-CH), 8.67 (m, 2H, p-CH), 8.91 (d, 4H, o-CH).
2,2-bis((4-methylpyridinium)methyl) propane-1,3-diol hexafluorophosphate (8). White solid (0.40 g, 69%); FT-IR (KBr) ν: 3601, 3040, 2981, 2855, 1580, 1564, 1450, 1425, 1310, 1168, 1019, 843 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 2.64 (s, 6H, CH3), 3.13 (s, 4H, OH-CH2), 4.69 (s, 4H, N-CH2), 5.57 (s, 2H, OH), 8.03 (m, 8H, Ar-H).
2,2-bis((1-methylpiperidinium)methyl) propane-1,3- diol hexafluorophosphate (9). White solid (0.18 g, 31%); FT-IR (KBr) ν: 3605, 2987, 2886, 1500, 1445, 1423, 1306 1164, 1010, 845 cm−1. 1H NMR (DMSO-d6, 500 MHz) δ: 3.02 (s, 6H, CH3), 3.40 (m, 12H, CH2-CH2-CH2) 3.79 (s, 4H, OH-CH2), 3.90 (d, 4H, N-CH2), 4.23 (m, 4H, N-CH2), 4.55 (m, 4H, N-CH2), 5.63 (d, 2H, J = 4 Hz, OH).
4.3. General Procedure for Suzuki Reaction in H2O and Recovery of Catalyst
In a typical experiment, a mixture of ligand (4.0 mol%) with PdCl2 (2.0 mol%) in water (4.0 mL) was added 4- nitrobromobenzene (0.19 g, 1 mmol) and phenyl boronic acid (1.2 mmol), and then K2CO3 (0.28 g, 2 mmol) was added. The mixture was reacted at 30˚C. On completion monitored by TLC and GC, the reaction mixture was extracted with ethyl ether (10 mL × 3). The combined organic extracts were washed with 10% NaOH (10 mL × 3), dried with Na2SO4, filtered and concentrated under vacuum to give the desired product. MS (GC/MS) m/z: 154 (M+, 100%), 77 (10%), 51 (8%). 1H NMR (500 MHz, DMSO-d6, ppm): δH 7.40 - 7.60 (m, 10 H, ArH).
The remaining liquid phase composed of PdCl2, ligand and the formed salt of KCO3 was used directly without further treatment for the next run through treated with 4- nitrobromobenzene (0.19 g, 1 mmol), phenyl boronic acid (0.18 g, 1.5 mmol), K2CO3 (0.28 g, 2 mmol) again.
5. Acknowledgements
We acknowledge the Natural Science Foundation of China (Grant 20676033), China Postdoctoral Science Foundation (Grant 20070410169) and Shanghai Leading Academic Discipline Project (Project Number: B507) for financial support.
NOTES