1. Introduction
With increasing use of gamma rays in different purposes such as medicine and industry along with the wide spread of nuclear power plants, it has now become necessary to study attenuation properties of various materials and compounds.
Major mass of nuclear radiation shield consists of different concretes with different densities, but water contents in concretes add uncertainty in calculation of shielding properties and moreover they are also opaque to visible light [1]. Glass materials are one of the possible alternatives because they are transparent to visible light and their properties can be adjusted by preparation techniques [1,2]. Borate glasses containing heavy metal oxides are thoroughly studied and the results show that they have potential applications in radiation shielding [3-6].
Many publications compiled the data of the mass attenuation parameter for different materials [7-10]. Berger and Hubbell [11] developed a computer program called the XCOM to evaluate cross-sections and attenuation coefficients for elements, compounds and mixtures for photon energies from 1 keV to 100 GeV. This program was transformed to the Windows platform by Gerwed et al. [12,13]. This Windows version is called winXCOM. In a previous work, Singh et al. [2] have determined the attenuation properties of xZnO·2xPbO·(1 − 3x)B2O3 (x = 0.1 - 0.26) glasses at photon energies 511, 662, 1173 and 1332 keV. It is pointed out that these glasses deserve great interest in radiation shielding applications.
The aim of the present work is to investigate gamma interactions with ZnO-PbO-B2O3 glasses by measuring the mass attenuation coefficient in a broad energy range making use of the 662, 1173, 1332 and 2614 keV gamma lines. The attenuation properties of the hard 2614 keV gamma line are of special importance because the total γ-rays emitted from the interaction of the reactor collimated fast and thermal neutrons beam with the ordinary shielding material such as magnetite concretes lies between 2 - 8 MeV and γ-rays of energy within 2 - 4 MeV are the most predominant. From the experimental results of the attenuation coefficients, the values of the mean free path (MFP) and half value layer (HVL) have been obtained. A comparison between the mass attenuation coefficients at different gamma ray energies with the corresponding predictions of the winXCOM computer program has been performed.
The relation between the increase of the PbO weight fraction and hardness of glass has been tested. Additionally, the present work includes a preliminary experimental analysis of the UV absorption bands in terms of intensity and position.
2. Theoretical Aspects
It is well known that a parallel beam of monoenergetic gamma-ray or X-ray photons is attenuated in matter according to the Lambert-Beer law:
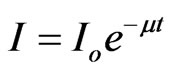 (1)
(1)
where Io and I are the incident and transmitted intensities, t is the thickness of the absorbing medium and m is the linear attenuation coefficient.
For photons in an attenuating medium, the mass attenuation coefficient (m/r) is given by
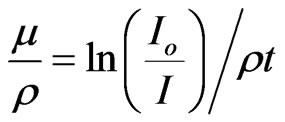 (2)
(2)
where r is the density of the absorbing material.
The mean free path (MFP) and the half value layer (HVL) can be evaluated using the following relations:
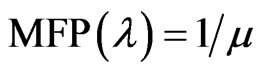 (3)
(3)
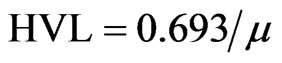 (4)
(4)
3. Experimental Procedure
Glass batches were prepared from chemically pure raw materials. The orthoboric acid (H3BO3) was used to obtain boron oxide B2O3. The chemicals were thoroughly mixed and porcelain crucibles containing the batch were placed in electrically-heated furnaces and kept at a temperature of 1050˚C for two hours under normal atmospheric conditions. The crucibles were removed from the furnaces and rotate through to produce homogeneous glass.
The glass was cast at a preheated stainless steel molds with dimensions 8 × 6 × 1 cm. All the glasses were properly annealed at 400˚C temperature in a muffle furnace. Then the muffle furnace was left to cool at a rate of 30˚C/hours down to room temperature. Annealing process was done to avoid and minimize the stresses and strains, which may be found in the final glass product.
The density of the prepared glasses was measured by indirect method based on Archimedes’ principle using xylene as immersion liquid.
The hardness was measured by a shore durometer (Instron Company United States). The samples were ground and highly polished before absorption measurements. Optical absorption was measured using a Shimadzu spectrophotometer (Japan) covering the wavelength range 200 - 1100 nm.
A 60CO gamma cell was used as a gamma-ray source for irradiation at room temperature with a dose rate of 1.5 Gy/sec. The glass samples were placed in the gamma cell in the manner that each sample was subjected to the same gamma dose. Attenuation measurements were performed using a narrow-beam transmission method.
The experimental arrangement consists of Hyper Pure Germanium (HPGe) detector with relative efficiency »10% relative to a 3"× 3" NaL (TI) detector, active volume 62.3 cm3 and resolution 1.8 keV at 1.33 MeV g-line. The detector was coupled through an amplifier to the computer with MCA plug-in card. Because of the sensitivity of HPGe detector, it is usual to shield them from the environment. Therefore, lead shield of thickness 5 cm is used in this study. The energy calibration of the spectrometer was performed using the well-known standard sources (22Na, 60CO, 57Co, 232Th and 241Am).
4. Results and Discussion
Chemical composition, densities and hardness for the prepared glass samples are given in Table 1. From this table, it is shown that by increasing the lead oxide content instead of boron oxide, the density of the glass batch increases. This may be attributed to the similarity of the position on the glass matrix. i.e. lead, which is the most absorbing component in the compound, takes the same positions in the glass network as boron; not in the intersects of the network as modifiers [14]. Moreover, it is obvious from the data that the hardness increases monotonously with decreasing B2O3 and replacing it by PbO. This may be due to that the strength of B-O bonds in these glasses is considerably weak compared with Pb-O bonds.
Experimental and theoretical values of mass attenuation coefficients are shown in Figure 1, where a good agreement between them is obviously noticed. The theoretical values of mass attenuation coefficients have been calculated using winXcom computer program [11]. Also, Figure 1 shows that, mass attenuation coefficients increase linearly with the weight fraction of PbO and decrease by increasing gamma-ray energy. In all experimental results, the estimated experimental uncertainties are about ±3%.
Table 2 shows the MFP and the HVL as a function of PbO content, at different g-ray energies, for the lead borate glasses. As expected, the MFP decreases with increasing PbO content, which is the most absorbing one of the three compounds. Similarly, the HVL decreases with increasing weight fractions of PbO in this glass system. This may be due to the increase in densities of glass samples and the higher values of mass attenuation coefficients. Iron con-
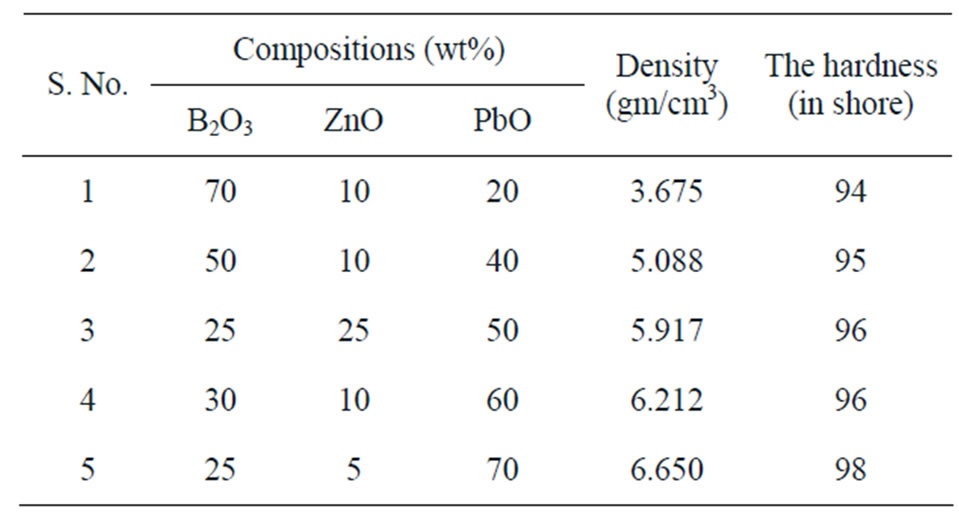
Table 1. Chemical compositions of glass samples.
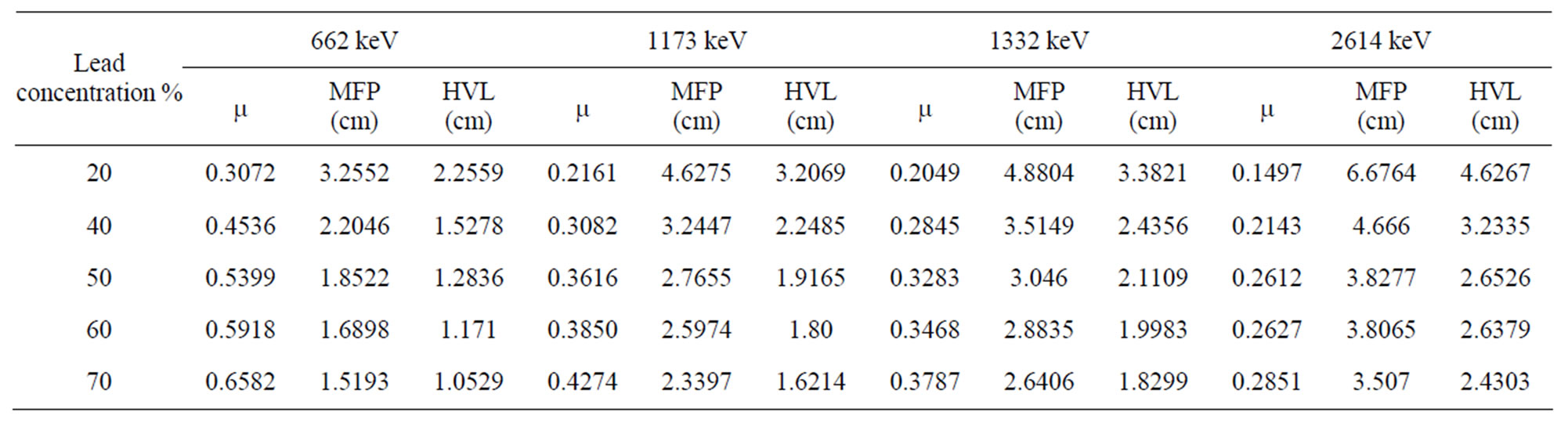
Table 2. Mean free path (MFP) and half value layer (HVL) for the glass samples at different gamma ray energies.
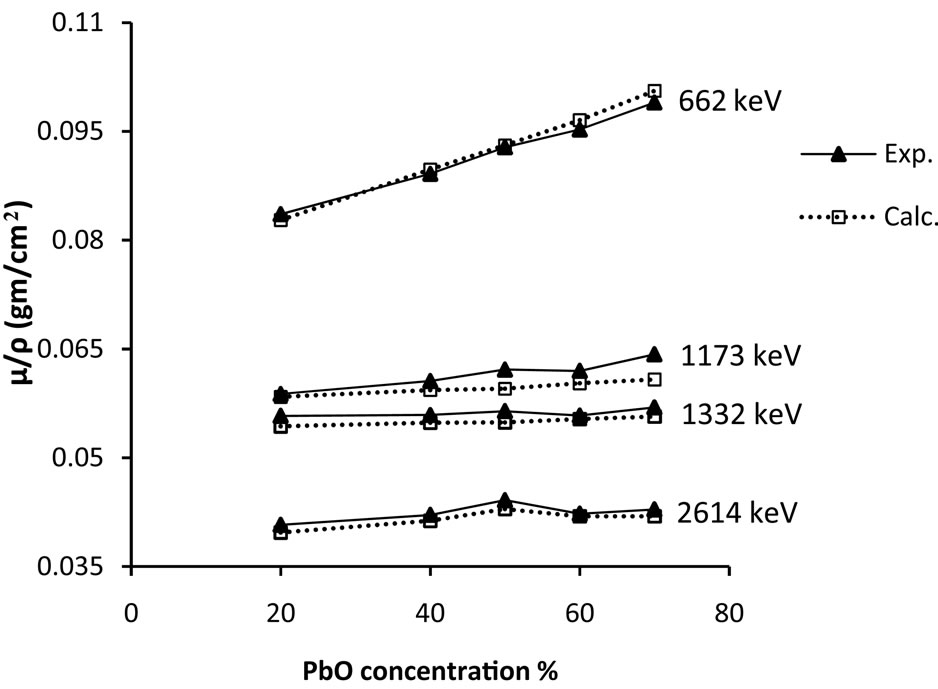
Figure 1. Mass attenuation coefficient for different lead concentration of the glass batches.
crete’s HVL values have been included in Figure 2 for comparison. The results indicate that lead glasses have higher values of mass attenuation coefficients and smaller values of HVL than standard shielding concretes.
Figure 3 shows the ultraviolet (UV)-visible absorption spectra of the undoped base triborate glass before and after different irradiation doses. The absorption spectra of the unirradiated base reveal an UV absorption peaks at about 235 and 280 nm with no visible bands. Irradiation of this glass to a dose 2 MR of gamma rays causes an increase in the UV bands intensity and the first band splits to two peaks. The visible region shows a broad band at about 540 nm. By increasing the gamma irradiation dose the UV bands intensities grow where the first band increase is larger than that of the second UV band. It is also noticed that the intensity of the visible band grow as a direct consequence to the irradiation dose. The present absorption spectra of the undoped base triborate glass is similar to that obtained previously by El-Batal et al. [15]. In their work, the UV-visible absorption spectrum belongs to the undoped base lithium diborate. Therefore, this similarity means that Li contribution to the fore mentioned absorption spectra is negligible. As a matter of fact, “Li” is the smallest metal element that characterized by strong co-